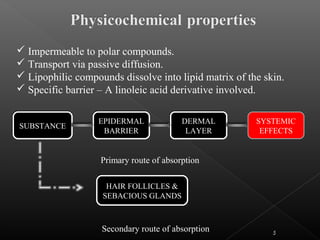
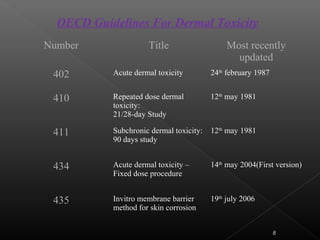
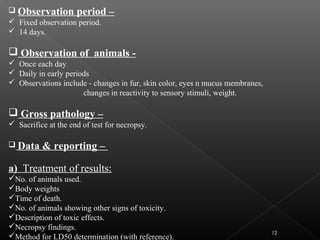
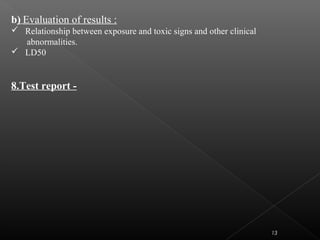
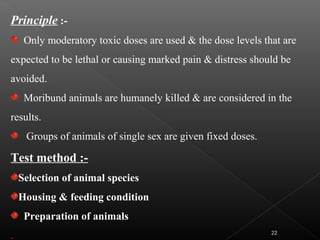
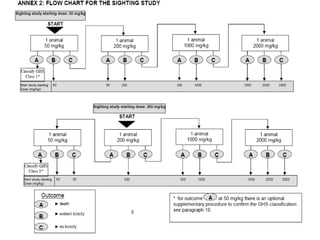
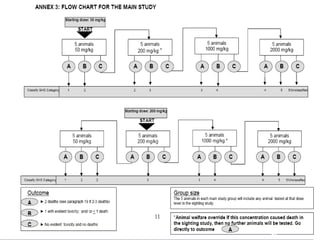
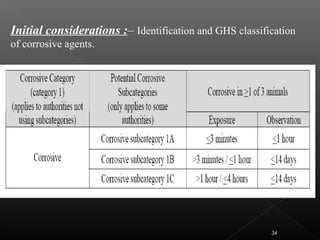
This document discusses various aspects of dermatotoxicity testing including skin structure and function, dermatotoxic reactions, OECD testing guidelines, and specific dermatotoxicity tests. It summarizes common dermatotoxicity tests including acute dermal toxicity tests, repeated dose dermal toxicity tests lasting 21-28 days, subchronic dermal toxicity tests lasting 90 days, and in vitro tests for skin corrosion. Testing procedures, observations, data collection, and reporting requirements are outlined for each test.
![Presented by:
Chaudhari Sandip
M.Pharm[sem-II]
Department of Pharmacology
R. C. Patel Institute Of Pharmaceutical Education &
Research, Shirpur](https://image.slidesharecdn.com/oecd-170202064950/75/OECG-Dermatotoxicity-testing-1-2048.jpg)