The document summarizes several OECD guidelines for dermal toxicity testing:
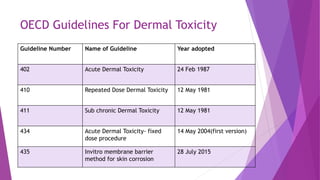
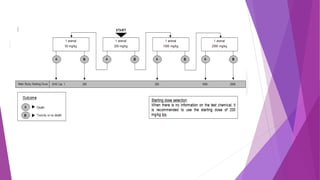
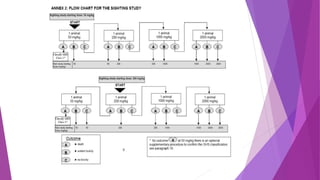
- Guideline 402 describes methods for acute dermal toxicity testing using rats. Animals are exposed to test chemicals at fixed doses and observed for 14 days.
- Guidelines 410, 411, and 434 describe methods for repeated dose dermal toxicity testing over 21/28 days, 90 days, and other durations to evaluate sub-chronic effects. Rats, rabbits or guinea pigs are exposed daily and observed for signs of toxicity.
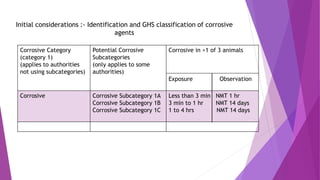
- Guideline 435 describes an invitro membrane barrier method for evaluating skin corrosion potential without using live animals.