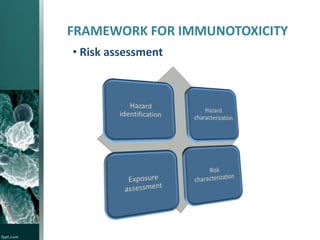
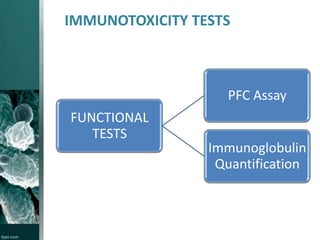
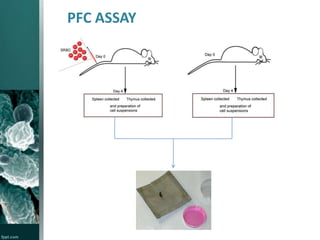
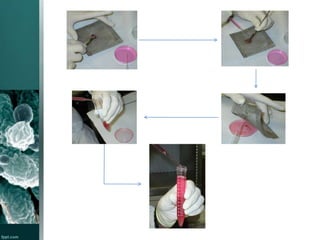
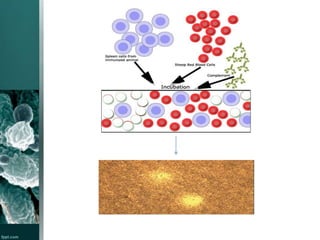
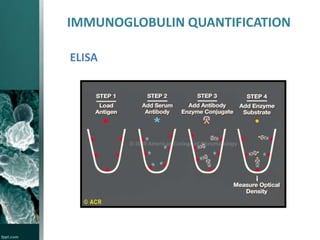
This document provides an overview of immunotoxicity testing procedures. It discusses the framework for immunotoxicity risk assessment, including hazard identification, characterization, exposure assessment, and risk characterization. General terminology related to immunotoxicity is defined. Test procedures are outlined, including animal selection, dosing, observation, and functional tests like the plaque forming cell assay and immunoglobulin quantification. The document also discusses additional tests that may be conducted depending on evidence, such as host resistance assays, hematology, and histopathology examinations. Test data is to be treated and reported according to good laboratory practice standards.