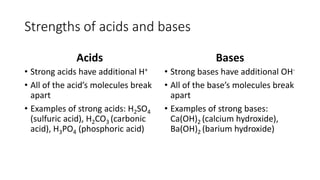
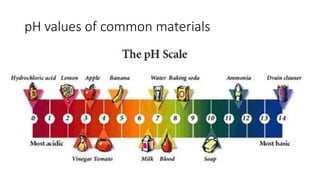
This document discusses acids and bases, including their properties and uses. It defines acids as compounds that increase hydronium ions in water and bases as compounds that increase hydroxide ions in water. Examples of acids include H2SO4 and HCl, while examples of bases include NaOH and Ca(OH)2. Acids and bases have similar properties such as being corrosive or changing the color of litmus paper. They have many industrial and household uses like making paper, detergents, and antacids. The document also explains how strong acids and bases fully dissociate in water and the neutralization reaction between acids and bases.