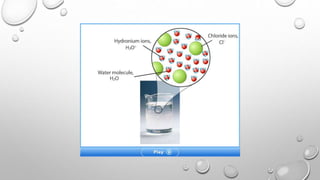
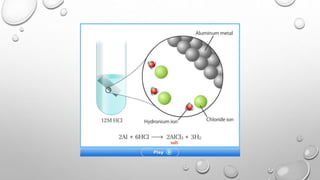
This document discusses acids and bases. It defines acids as compounds that increase hydronium ions in water and bases as compounds that increase hydroxide ions in water. Strong acids and bases fully dissociate in water, while weak acids and bases only partially dissociate. The pH scale is used to measure how acidic or basic solutions are. Neutralization reactions occur when acids and bases react, forming water and salts. Common acids and bases have various industrial and household uses.