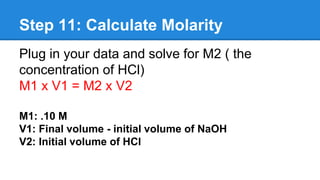
This document provides instructions for performing an acid-base titration experiment. The experiment involves titrating a solution of sodium hydroxide (NaOH) of known molarity with hydrochloric acid (HCl) of unknown molarity. Phenolphthalein indicator is used to detect the endpoint of the reaction, which is the point at which the moles of acid equal the moles of base. By measuring the volumes of the NaOH and HCl solutions before and after titration, the molarity of the HCl solution can be calculated using the titration equation. The document outlines the 12 steps of the experiment, which include gathering materials, adding solutions, titrating with stirring until the endpoint is