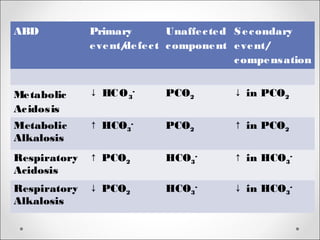
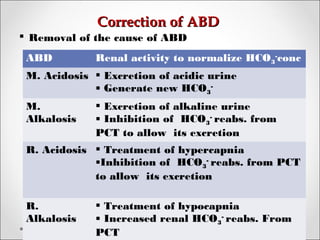
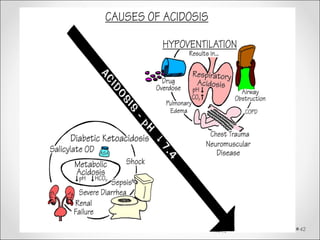
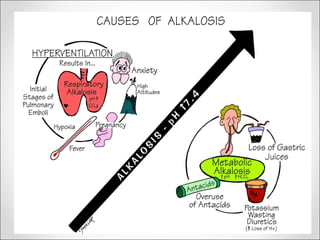
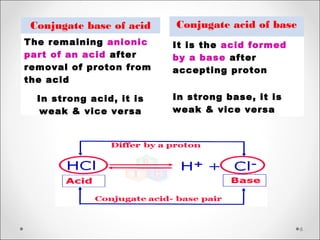
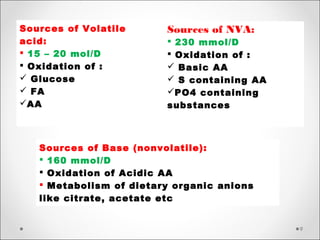
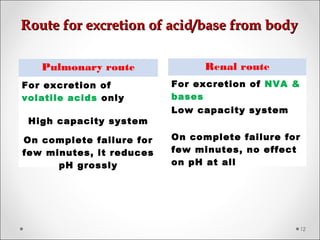
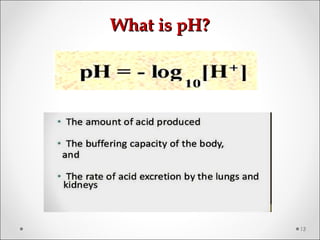
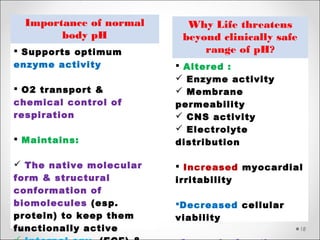
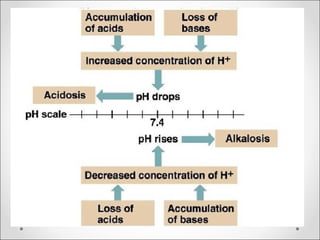
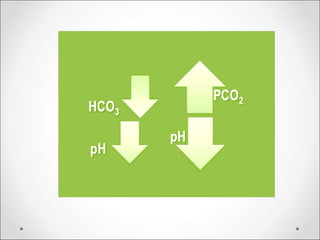
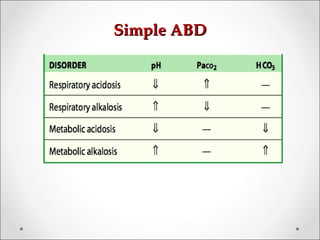
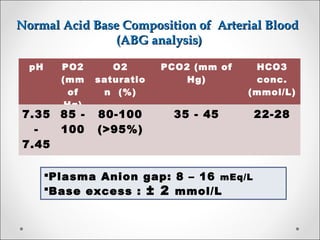
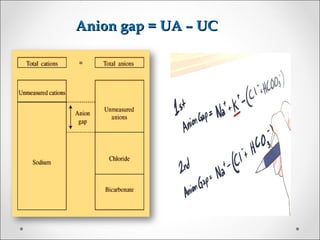
The document discusses acid-base balance, detailing definitions, types of acids and bases, and sources of acids in the body, including both exogenous and endogenous sources. It explains how pH is regulated through chemical buffer systems, pulmonary, and renal routes, and emphasizes the importance of maintaining normal body pH for optimal enzyme activity and overall physiological function. Additionally, it addresses acid-base disorders, their causes, parameters for assessment, and compensation mechanisms in response to abnormal conditions.









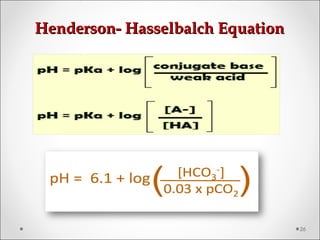
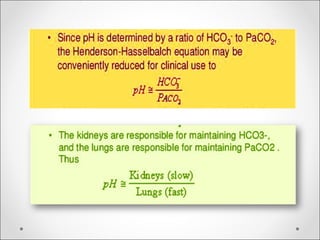

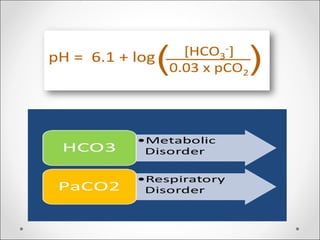


![BE = [HCOBE = [HCO33
--
] p – [HCO] p – [HCO33
--
]std]std](https://image.slidesharecdn.com/acidbasebalanceabdformdms-180424035138/85/Acid-base-balance-acid-base-disorder-37-320.jpg)