Embed presentation
Downloaded 134 times

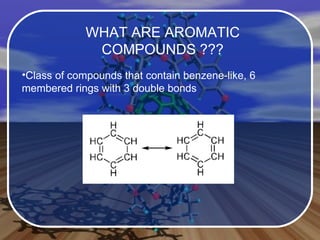
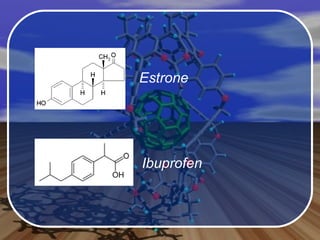
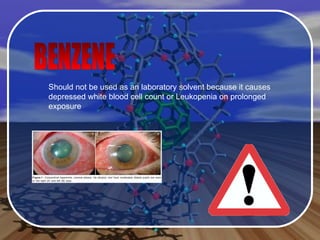
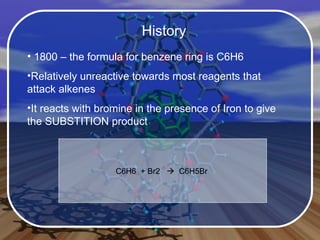




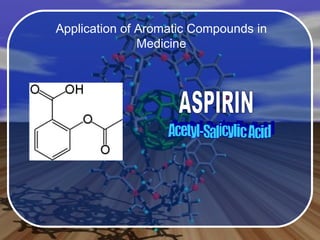
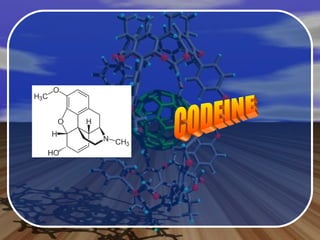
Aromatic compounds contain benzene-like, 6-membered carbon rings with 3 double bonds. They are unusually stable and undergo electrophilic substitution reactions. Many drugs contain aromatic compounds, such as aspirin, codeine, and ibuprofen, which are used for pain relief and medicine.










