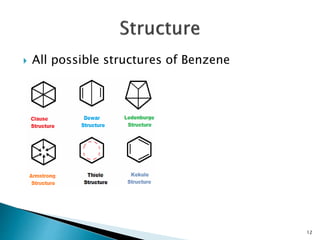
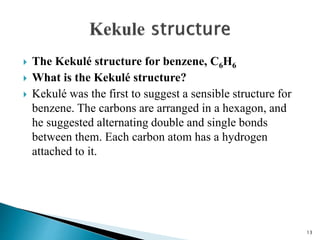
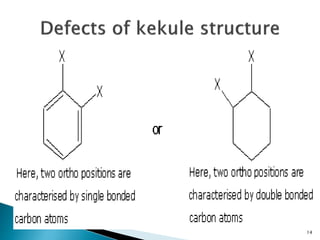
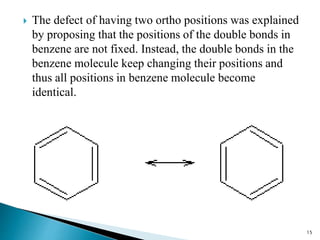
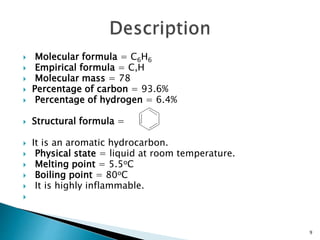
The document provides a detailed overview of benzene, including its history, structure, properties, and applications. It highlights benzene's chemical formula (C6H6), its classification as an aromatic hydrocarbon, and its uses in the manufacturing of various products like nylon, drugs, and explosives. Additionally, it discusses potential exposure risks and health effects related to benzene vapors and contaminated water.


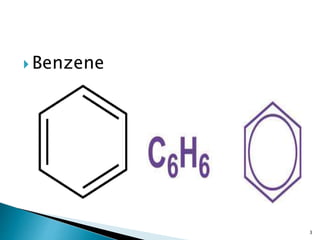





![ Benzene is a natural constituent of crude
oil and is one of the
elementary petrochemicals . Because of the
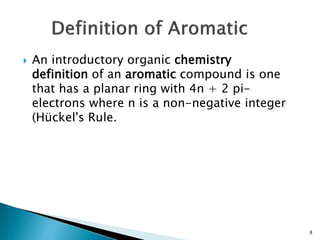
cyclic continuous pi bond between the carbon
atoms, benzene is classed as an aromatic
hydrocarbon, the second [n]-annulene ([6]-
annulene).
Benzene is a colorless and highly flammable
liquid with a sweet smell, and is responsible
for the aroma around petrol stations.
11](https://image.slidesharecdn.com/organic6thsemester-170921043517/85/Basic-Introduction-Of-benzene-11-320.jpg)