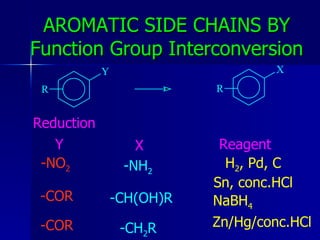
This document discusses retrosynthetic analysis approaches for aromatic compounds. It covers aromatic electrophilic and nucleophilic substitution reactions, including addition of cationic synthons, Friedel-Crafts alkylation and acylation, and substitution of diazonium salts. It also discusses aromatic side chain transformations through functional group interconversion and nucleophilic aromatic substitution of halides.