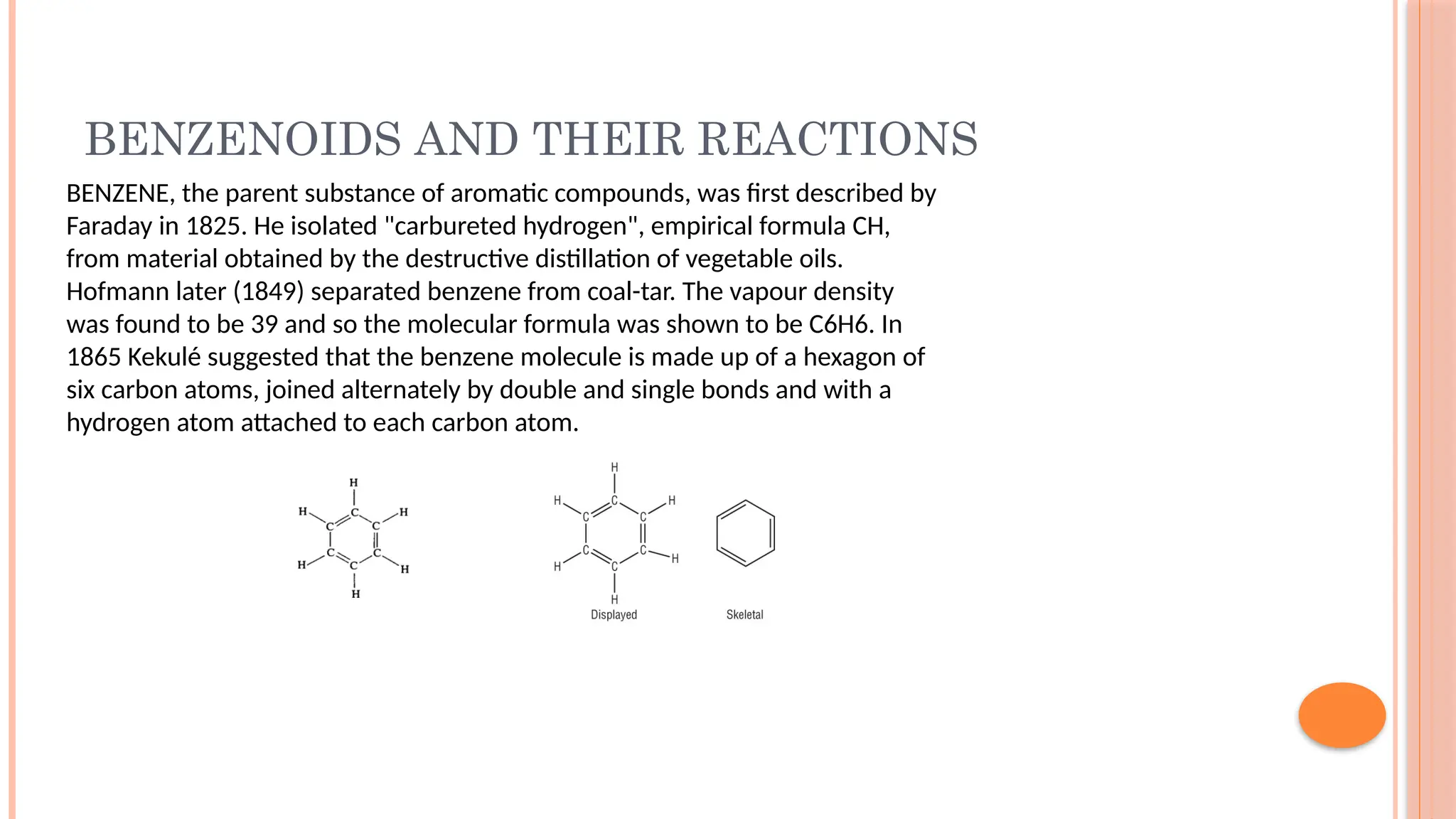
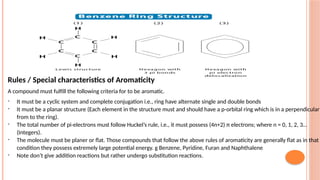
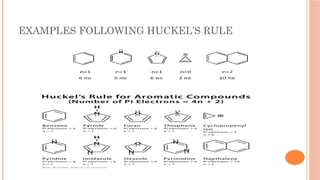
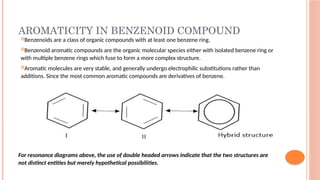
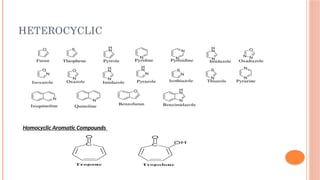
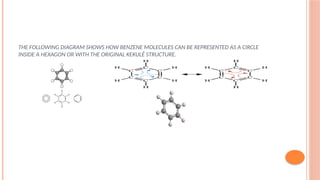
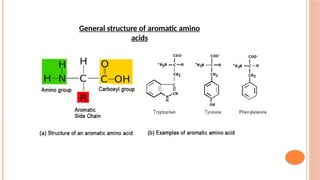
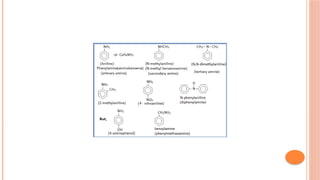
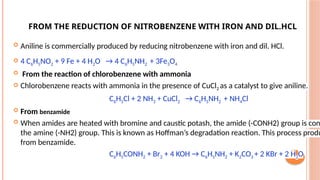
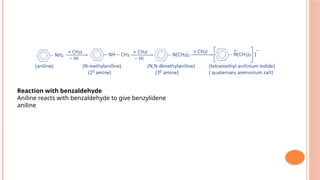
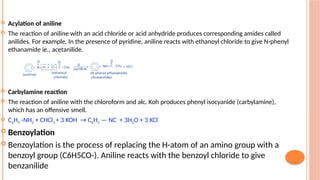
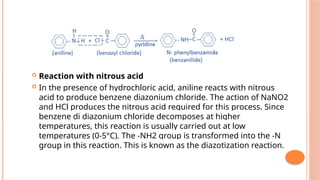
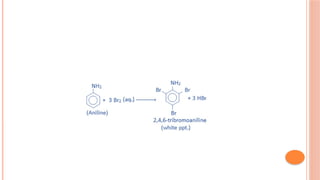
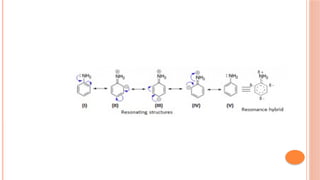
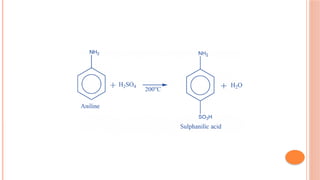
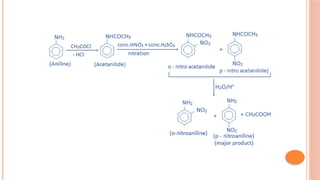
The document discusses benzenoids, aromatic compounds, and their reactions, starting with the historical discovery of benzene and the concept of aromaticity, which describes the unique stability of cyclic, planar molecules with delocalized pi electrons. It covers the synthesis and classification of aromatic amino acids as well as the shikimate pathway, detailing their roles in plant metabolism and various applications in drugs, sweeteners, and industrial chemicals. The text also outlines the chemical properties and reactions of aniline, including electrophilic substitutions and its use in producing various products like pesticides and plastics.