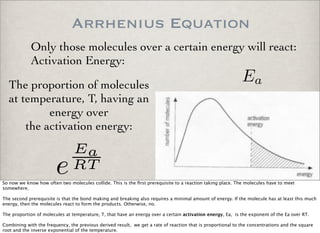
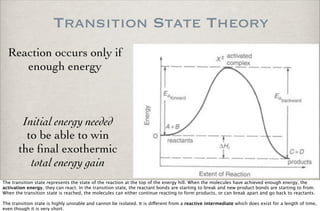
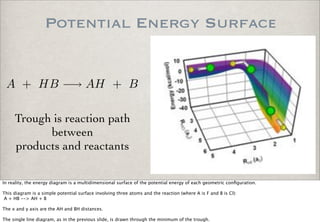
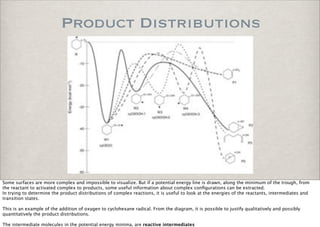
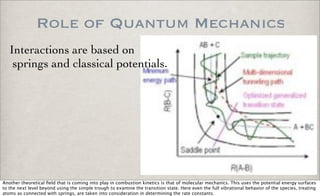
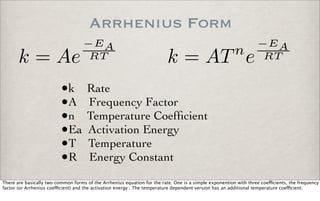
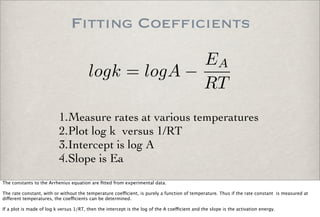
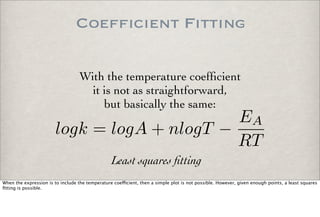
This document discusses chemical kinetics, focusing on reaction rates, the Arrhenius equation, and transition state theory. It describes how collision theory explains the frequency of molecular collisions and how temperature and activation energy influence reaction rates. The use of quantum mechanics and potential energy surfaces is highlighted for more accurate calculations of reaction kinetics and the determination of rate constants from experimental data.








![Arrhenius Equation
But what happens with more complicated
with sets of reactions?
(as in combustion)
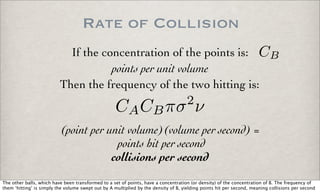
Rate of products production:
A + B −→ products
d(products)
dt = k[A][B]k = Te
Ea
RT
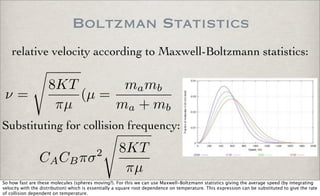
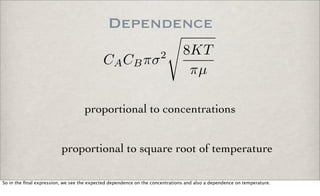
From the discussion of bimolecular collision theory, we have a rate equation for a single reaction of species A and species B
combining to form products.
d[products]/dt = k[A][B]
where the rate constants has a temperature dependence:
k = T**n exp(Ea/RT)
But combustion mechanisms consist of many of such reactions, of di!erent orders (number of reactants). The following
discussion looks at dealing with the more complicated cases and simplifications that can be made.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-25-320.jpg)
![First Order
A −→ BUnimolecular:
d[A]
dt
= −k[A]
d[B]
dt
= k[A]
Reversible Unimolecular: A ←→ B
d[A]
dt
= −kf [A] + kr[B]
d[B]
dt
= kf [A] − kr[B]
Let us look at the rate equations for unimolecular transformations between species A and species B.
For the first case of non-reversible transformation of A to B, the rate of production is simply minus the rate times its concentration, i.e. the
concentration is decreasing. The increase of B is also dependent on how much A there is.
A reversible reaction of one reactant and one product is also first order.
Thought as two independent reactions, one in the forward and one in the reverse, we get the expected equations.
But now, for example, the molecule A has two sources and sinks. In the forward direction, A is being converted to B at the rate of -k[A] (note
the negative sign). However, at the same time, it is being created by B being converted to A at the rate of - k[B].
Thus the rate of production of A is the sum of the forward and reverse directions: - k_f[A] + k_r[B]](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-26-320.jpg)
![Bimolecular Reactions
A + B −→ CRecombination:
d[A]
dt
= −k[A][B]
d[B]
dt
= −k[A][B]
d[C]
dt
= k[A][B]
Just as in the previous case, the total rate of production is the sum of all the sources and sinks of the molecular species.
For the forward reaction, there is only one source of each rate of production, namely plus or minus k[A][B].](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-27-320.jpg)
![Bimolecular: Reversible
d[A]
dt
= −kf [A][B] + kr[C]
d[B]
dt
= −kf [A][B] + kr[C]
d[C]
dt
= +kf [A][B] − kr[C]
A + B ←→ C
However, for the reversible case, once again, there is the e!ect of both sides of the reaction.
In general, to determine the rate of production of the species
- Collect all the reactions involving that species.
- Add all the rates (with concentrations depending on order) producing the species
- Subtract all the rates that are consuming the species.
For a complex mechanism, one has a set of di!erential equations, one for each species. On the left hand side is the di!erential and on the right
hand side a polynomial expression in terms of rates and species concentrations.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-28-320.jpg)
![Rate of Production
d[H]
dt = +2k1[H2] H2 + M −→ H + H + M
+k2[H2][O2] H2 + O2 −→ HO2 + H
−k3[H][O2] H + O2 −→ O + OH
+k4[O][H2] O + H2 −→ H + OH
+k5[H2][OH] H2 + OH −→ H2O + H
−2k7[H]2
H + H + M −→ H2 + M
−k9[H][O] H + O + M −→ OH + M
−k10[H][OH] H + OH −→ H2O
A more typical case, for even a simple example like one part of the hydrogen mechanism, the rate of production of just one species can be
quite complex. One can see that the set of di!erential equations that must be solved, for even a simple molecule like hydrogen, can be quite
complex. Each species di!erential is equal to a complex polynomial.
So n complex di!erential equations must be solve to determine the time evolution of a species within a given mechanism. This should give an
indication why kinetics is a di"cult computational task when dealing with, for example, reactive flows.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-29-320.jpg)

![Quasi-First Order
A + B −→ C
Assume [B] is constant
(pool approximation)
Often, for example, [B] >> [A]
d[A]
dt = −k[A][B] = −k [A]
d[B]
dt = −k[A][B] = −k [A]
d[C]
dt = +k[A][B] = +k [A]
where: k' = k[B]
In a bimolecular reaction, sometimes one of the reactants, for example B, has a comparatively large concentration. The
consequence of this is that it total concentration does not change much, the pool approximation. This simplifies the second
order equations to a quasi first order equations.
For example, suppose that [C] is 1000 moles/liter and that [B] is 1 mole/liter. Even if the reaction, A + B --> C, went to
completion meaning all the B would be used up, [C] would still be fairly close to 1000, i.e. with some assumptions, 999 moles/
liter.
This means that $k[B]$ is relatively constant and only [A] is changing significantly. Thus the rate equation can be thought of a
being basically first order in A.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-31-320.jpg)
![Quasi-Steady State
allows algebraic solving reduces the complexity
(after a rapid initial buildup)
It reaches an equilibrium.
The concentration of a species does not change
d[A]
dt
= 0
If molecule is in steady state, then that means that its concentration is not changing. This means that the consumption and
depletion rates are the same. It is important to note that molecule does indeed have a non-zero steady state concentration.
Mathematically, a molecule is in steady state when d[A]/dt = 0. This fact can be very useful in simplifying the set of di!erential
equations of a reactive system. For each molecule in steady state, a di!erential equation is eliminated and is replaced by a purely
algebraic equation (which is simpler to solve).](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-32-320.jpg)
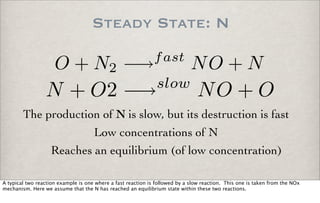
![Steady State: N
Note that it is not zero..
d[N]
dt
= 0 = k1[O][N2] − k2[N][O2]
[N]ss =
k1[O][N2]
k2[O2]
O + N2 −→fast
NO + N
N + O2 −→slow
NO + O
solving
Setting d[N]/dt to zero, leaves an algebraic equation where [N] can be solved for. The computations show that first, the steady
state concentration of N is non-zero and is dependent on [O], [N2] and [O2].
More assumptions have to be made to solve the system completely or to be able to make some computations.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-34-320.jpg)
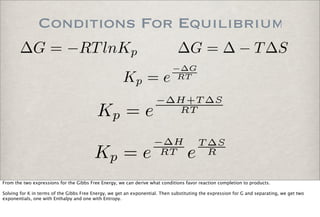
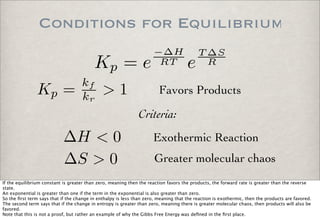
![Equilibrium Constant
A + B ←→ C + D
kf [A][B] = kr[C][D]
0 = d(products)
dt = d(reactants)
dt = kf [A][B] − kr[C][D]
Under Equilibrium Conditions
Kc =
kf
kr
= [C][D]
[A][B]
Consider the reversible bimolecular reaction involving the reactants A and B and the products C and D.
Under equilibrium conditions, the rate of production of the reactants is the same as the rate of production of the products.
Setting the di!erentials equal, we get an expression relating the
forward and reverse rate constants and concentrations of the reactants and products.
Collecting the rate terms on one side and the concentration
terms on the other side, we have the expression for
a defined quantity, the Equilibrium Constant.
The equilibrium constant gives the ratio of the reactant concentrations to the product concentrations. For example, a large
equilibrium constant means that the reaction goes close to completion and a significant portion of the species went to products.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-35-320.jpg)



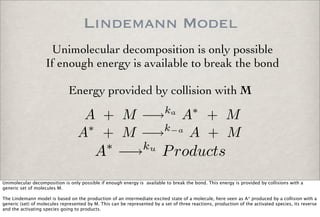
![Lindemann Equations
d[Products]
dt
= ku[A∗
]
Quasi-steady state of activated species
d[A∗
]
dt
= 0 = ka[A][M] − k−a[A∗
][M] − ku[A∗
]
[A∗
] =
ka[A][M]
k−a[M] + ku
d[Products]
dt
= ku[A∗
] =
kuka[A][M]
k−a[M] + ku
From the system of three reactions, the rate of production of the activated species can be written. Since this is an intermediate species, and its
decay is very fast, we can assume that the species is in quasi steady state and from that we can derive an algebraic expression for the
concentration of this activated species:
[A*]= k_a[A][M]/(k(-a)[M] + ku)
which can be substituted back in the equation
for the production of the product species:
d[Products]/dt = k_u [A*] = k_u k_a[A][M]/(k(-a)[M] + ku)](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-42-320.jpg)
![Fall Off Limits
At high pressure, the rate go asymptotically to a constant, k(inf)
At low pressure, the rate has a constant slope, ka[A][M].
In the transition zone the line with the slope bends to the asymptotic constant.
The Lindeman model recognizes that some kinetic relationships cannot be represented with a single rate constant. There are
several expressions within combustion kinetics which take this e!ect into account.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-43-320.jpg)
![Fitting Low Pressure
Low Pressure Range:
2nd Order Kinetics
(concentration of collision partners is small)
k−a[M] << ku
d[Products]
dt
= ku[A∗
] =
kuka[A][M]
k−a[M] + ku
d[Products]
dt
= ka[A][M]
A∗
−→ku
Products
At low pressure, the concentration of collision partners is small and the conversion to products dominates. So the term with M disappears in
the denominator leaving the expression ka[A][B], meaning second order kinetics.
This produces the slope of the line dependent on M in the graph.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-44-320.jpg)
![Fitting High Pressure
k−a[M] >> ku
(first order kinetics)
d[Products]
dt
= ku[A∗
] =
kuka[A][M]
k−a[M] + ku
d[Products]
dt
=
kuka[A][M]
k−a[M]
= kinf [A]
A∗
+ M −→k−a
A + M
At high pressure, the M term is larger and the reverse dominates. Thus the term with M in the denominator dominates leaving first order
kinetics in the final expression.
This produces the flat ‘fall o!’ behavior shown in the graph.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-45-320.jpg)
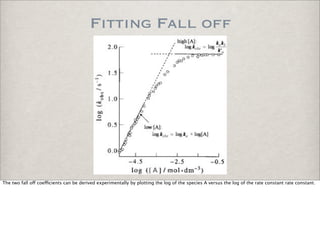


![Relation to Rate Constant
A −→ products
[A] = [A]0e−kt
d[A]
dt
= −k[A]
It takes an infinite amount of time to reach zero.
Not useful
How is the chemical time scale quantified?
How is the chemical time scale quantified?
Of course, the chemical time scale is directly influenced by the kinetic rate constant. The higher rate constant, the faster the process and thus
the smaller the chemical time scale. Thus there must be an inverse relationship.
However, for example, for a first order reaction, the reactant concentration decays exponentially, but it still takes an infinite amount of time to
reach zero. This is not a useful measure.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-49-320.jpg)
 =
[A]0
e
1
e
= .37 63% of the initial
[A](τchem) =
[A]0
e
= [A]0e−kτchem
τchem =
1
k
Since the time scale is a relative concept, it is not entirely important exactly at what point we decide. By convention (this is also used in
electronics), the time scale is determined to be the point at which the concentration is 1/e of the original. How much time is needed to consume
about 63% of the original substance.
Solving the original first order equation we find that the first order chemical time scale is the inverse of the rate constant.](https://image.slidesharecdn.com/p05-chemicalkinetics-091018063344-phpapp02/85/P05-Chemical-Kinetics-50-320.jpg)