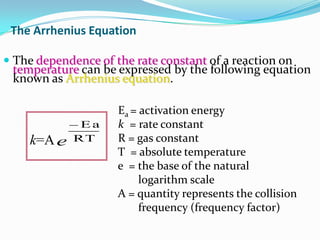
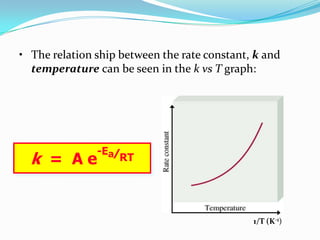
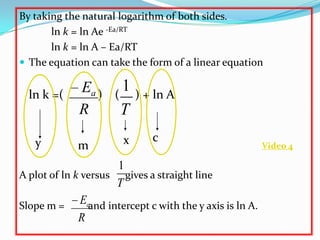
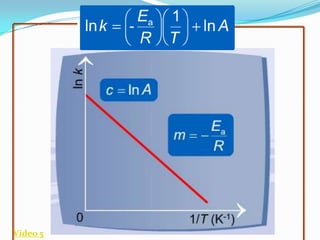
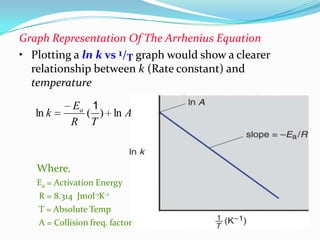
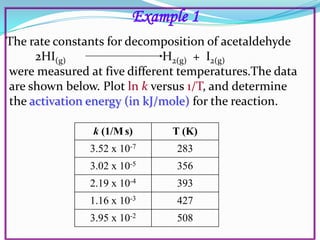
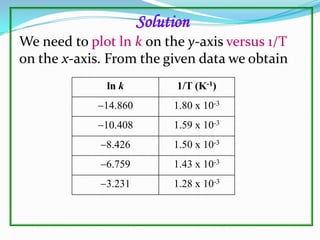
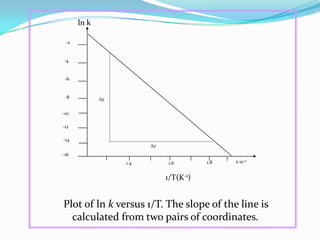
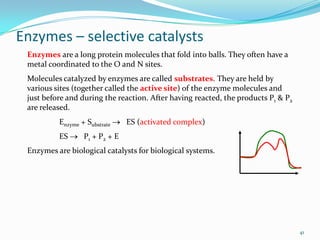
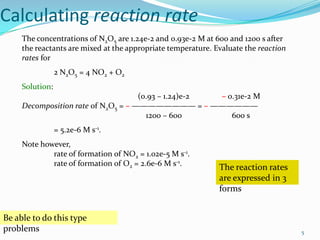
The document discusses reaction kinetics and methods for determining reaction rates. It defines reaction rate and explains how to express reaction rates using concentration changes over time. It also discusses calculating reaction rates from experimental data and determining the rate laws and orders of reactions. Integrated rate laws that relate concentration to time for first-order reactions are also covered, including calculating rate constants and half-lives. The Arrhenius equation, which relates reaction rate to temperature, is introduced.


![Reaction Rate Defined
Reaction rate: changes in a concentration of a
product or a reactant per unit time.
[]
Reaction rate = ——
t
concentration
[]
change
[]
t
t
3](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-3-320.jpg)
![Expressing reaction rates
For a chemical reaction, there are many ways to express the reaction rate. The
relationships among expressions depend on the equation.
Note the expression and reasons for their relations for the reaction
2 NO + O2 (g) = 2 NO2 (g)
[O2]
1
[NO]
1
[NO2]
Reaction rate = – ——— = – — ———— = — ———
t
2
t
2
t
Make sure you can write expressions for any reaction and figure out the
relationships. For example, give the reaction rate expressions for
2 N2O5 = 4 NO2 + O2
How can the rate expression be unique and universal?
4](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-4-320.jpg)
![Rate Laws
An equation that relates the rxn rate and the
concentration of reactants
Rate Determining Step
Slow
Rate = k[HBr][O2]](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-7-320.jpg)
![Rate Laws
If no mechanism is given, then…
2H2 + 2NO N2 + 2H2O
Rate = k[H2]2[NO]2](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-8-320.jpg)
![Rate Orders
0, 1st and 2nd order rates
Order is dependent upon what will yield a straight
line
1st order
ln [reactants]
[reactants]
0 order
1/[reactants]
2nd order](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-9-320.jpg)


![Calculating for k
A + 2B C
Rate = k[A][B]2
Experiment
Initial [A]
Initial [B]
Rate of
Formation of
C
1
0.20 M
0.20 M
2.0 x 10-4
M/min
2
0.20 M
0.40 M
8.0 x 10-4
M/min
3
0.40 M
0.40 M
1.6 x 10-3
M/min
What is the value of k, the rate
constant?](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-12-320.jpg)
![Calculating for k
Experiment
Initial [A]
Initial [B]
Rate of
Formation
of C
1
0.20 M
0.20 M
2.0 x 10-4
M/min
2
0.20 M
0.40 M
8.0 x 10-4
M/min
3
0.40 M
0.40 M
1.6 x 10-3
M/min
Rate = k[A][B]2
2.0 x 10-4 = k[0.20][0.20]2
2.0 x 10-4 = k(0.008)
k = 2.50 x 10-2 min-1 M-2](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-13-320.jpg)
![Practice
1. In a study of the following reaction:
2Mn2O7(aq) → 4Mn(s) + 7O2(g)
When the manganese heptoxide concentration was changed from 7.5 x
10-5 M to 1.5 x 10-4 M, the rate increased from 1.2 x 10-4 to 4.8 x 10-4. Write
the rate law for the reaction.
Rate = k[Mn2O7]2
2. For the reaction:
A+B→C
When the initial concentration of A was doubled from 0.100 M to 0.200
M, the rate changed from 4.0 x 10-5 to 16.0 x 10-5. Write the rate law &
determine the rate constant for this reaction.
Rate = k[A]2
Constant = 4.0 x 10-3 M/s](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-14-320.jpg)
![More Practice
New Rate = 8.1 x 10-5 M/s
3. The following reaction is first order:
CH3NC(g) → CH3CN(g)
The rate of this reaction is 1.3 x 10-4 M/s when the reactant
concentration is 0.040 M. Predict the rate when [CH3NC] = 0.025.
4. The following reaction is first order:
(CH2)3(g) → CH2CHCH3 (g)
What change in reaction rate would you expect if the pressure of
the reactant is doubled?
An increase by a factor of 2](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-15-320.jpg)
![Integrated Rate Laws
concentrations as functions of time
One reactant A decomposes in 1st or 2nd order rate law.
Differential rate law
Integrated rate law
– d[A] / dt = k
[A] = [A]o – k t
d[A]
– —— = k [A]
dt
[A] = [A]o e – k t or ln [A] = ln [A]o – k t
d[A]
– —— = k [A]2
dt
1
1
—— – —— = k t
[A]
[A]o
[A] conc at t
[A]o conc at t = 0
Describe, derive and apply the integrated rate laws
Learn the strategy to determine rate-law
16](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-16-320.jpg)
![Concentration and time of 1st order reaction
Describe the features of plot of [A] vs. t and ln[A] vs. t for 1st order reactions.
Apply the technique to evaluate k or [A] at various times.
[A]
ln[A
]
ln [A] = ln [A]o – k t
[A] = [A]o e – k t
t½
t
t
17](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-17-320.jpg)
![Half life & k of First Order Decomposition
The time required for half of A to decompose is called half life t1/2.
Since
[A] = [A]o e – k t
When
t = t1/2, [A] = ½ [A]o
Thus
ln ½ [A]o = ln [A]o – k t1/2
or
ln [A] = ln [A]o – k t
– ln 2 = – k t1/2
k t1/2 = ln 2 = 0.693
relationship between k and t1/2
Radioactive decay usually follow 1st order kinetics, and half life of an isotope is
used to indicate its stability.
Evaluate t½ from k or k from t½
18](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-18-320.jpg)

![st
1
order reaction calculation
N2O5 decomposes according to 1st order kinetics, and 10% of it decomposed in 30 s.
Estimate k, t½ and percent decomposed in 500 s.
Solution: Assume [A]o = [N2O5]o = 1.0, then [A] = 0.9 at t = 30 s or
0.9 = 1.0 e – k t
apply [A] = [A]o e– k t
ln 0.9 = ln 1.0 – k 30 s
– 0.1054 = 0 – k * 30
k = 0.00351 s – 1
t½ = 0.693 / k = 197 s apply k t ½ = ln 2
[A] = 1.0 e – 0.00351*500 = 0.173
Percent decomposed: 1.0 – 0.173 = 0.827 or 82.7 %
After 2 t½ (2*197=394 s), [A] = (½)2 =¼, 75% decomposed.
After 3 t½ (3*197=591 s), [A] = (½)3 =1/8, 87.5% decomposed.
Apply integrated rate law to solve problems
20](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-20-320.jpg)
![Molecularity of Elementary Reactions
The total order of rate law in an elementary reaction is molecularity.
The rate law of elementary reaction is derived from the equation. The order is the
number of reacting molecules because they must collide to react.
A molecule decomposes by itself is a unimolecular reaction (step);
two molecules collide and react is a bimolecular reaction (step); &
three molecules collide and react is a termolecular reaction (step).
O3
O2 + O
rate = k [O3]
NO2 + NO2
NO3 + NO
rate = k [NO2]2
Br + Br + Ar
Br2 + Ar*
rate = k [Br]2[Ar]
Caution: Derive rate laws this way only for elementary reactions.
21](https://image.slidesharecdn.com/chapter1-reactionkinetics-131209005149-phpapp01/85/Reaction-Kinetics-21-320.jpg)