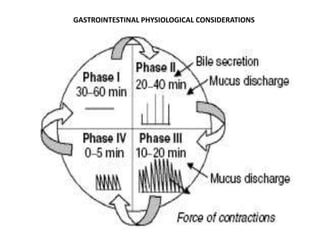
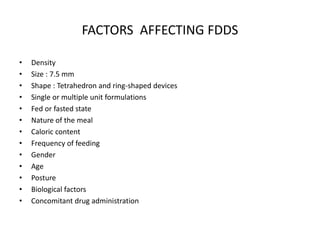
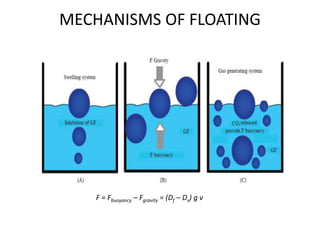
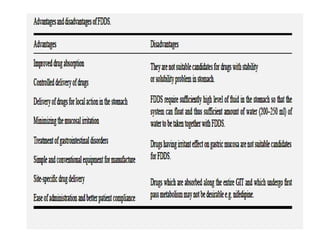
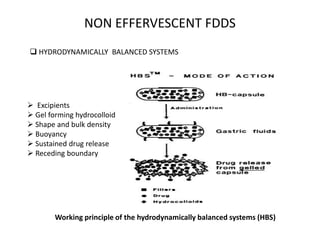
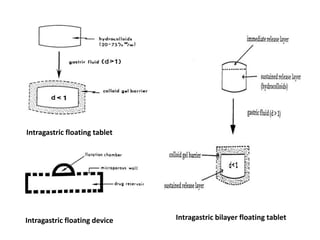
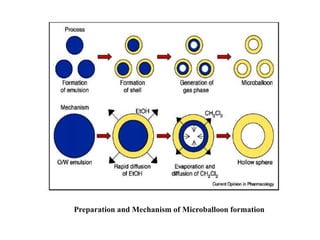
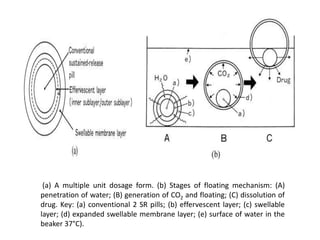
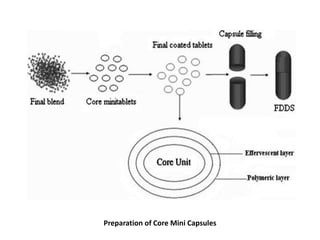
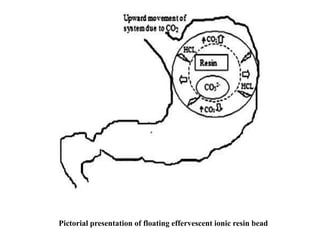
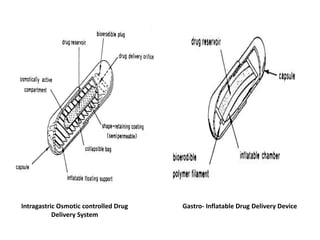
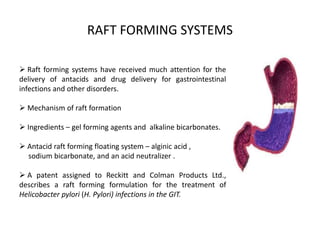
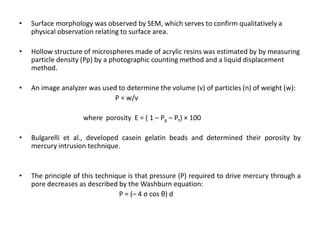
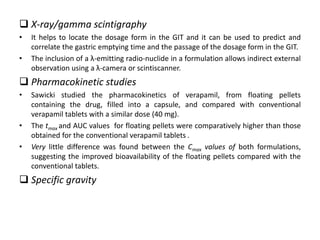
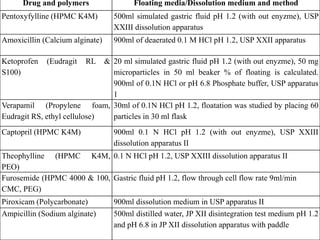
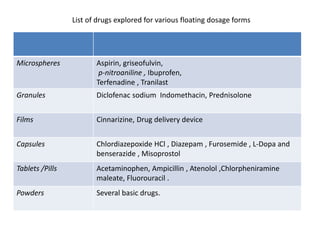
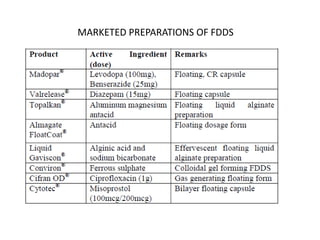
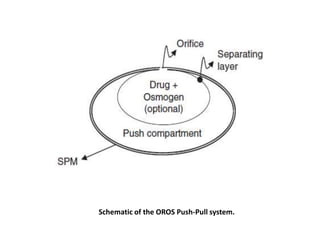
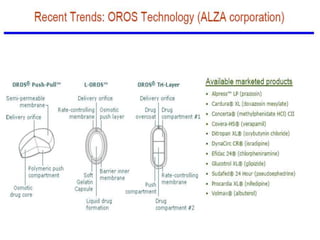
This document provides an overview of floating drug delivery systems. It begins with an introduction that describes how floating drug delivery systems can help modify gastric retention time and control drug release. It then discusses various factors that affect floating drug delivery systems and different mechanisms for achieving floatation. The document goes on to describe several types of non-effervescent and effervescent floating drug delivery systems that have been developed, including hydrodynamically balanced systems, hollow microspheres, alginate beads, and single-unit or multi-unit effervescent systems. It concludes by noting some applications and recent advances in floating drug delivery systems.