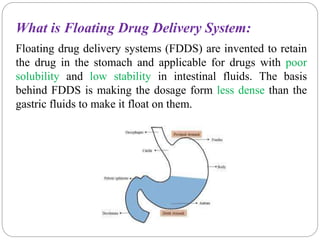
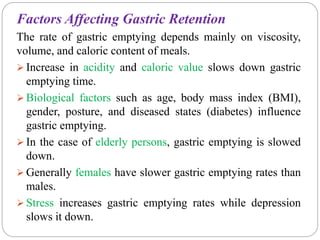
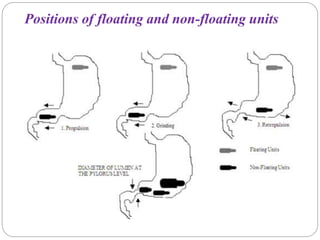
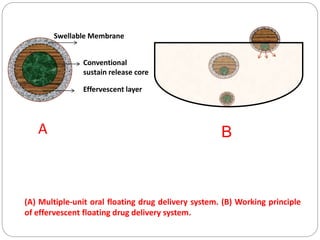
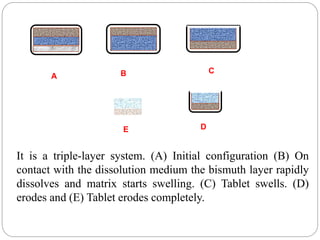
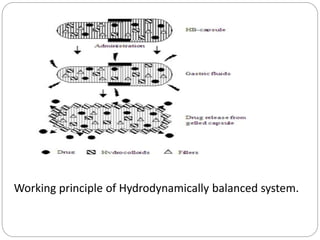
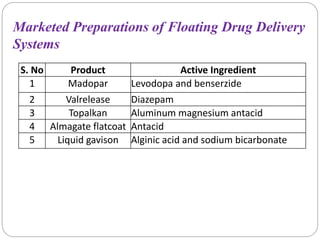
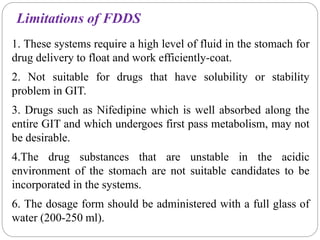
The document reviews floating drug delivery systems (FDDS), highlighting their mechanisms for improving drug bioavailability through gastric retention. It categorizes FDDS into effervescent and non-effervescent forms and discusses factors affecting gastric retention, as well as marketed preparations and evaluation parameters. The document also outlines the applications and limitations of FDDS for drugs with poor solubility and stability.