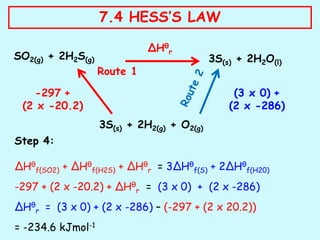
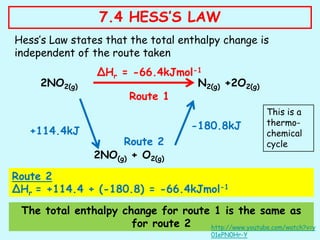
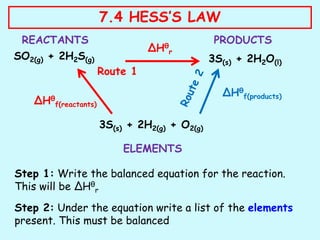
Hess's law states that the total enthalpy change for a reaction is independent of the pathway taken to get to the final products. It can be used to calculate enthalpy changes that cannot be measured directly through experimentation. The process involves writing balanced equations for each step of the reaction pathway and using standard enthalpy of formation values of reactants and products to determine the enthalpy change. An example is provided where the enthalpy change for the reaction SO2(g) + 2H2S(g) is calculated to be -234.6 kJ/mol by considering it as occurring in two steps and applying Hess's law.


![SO2(g) + 2H2S(g)
3S(s) + 2H2(g) + O2(g)
3S(s) + 2H2O(l)
ΔHθ
r
Route 1
ΔHθ
f(reactants)
ΔHθ
f(products)
REACTANTS PRODUCTS
ELEMENTS
Step 3:
ΔHθ
f[SO2(g)] = -297 kJmol-1
ΔHθ
f[H2S(g)] = -20.2 kJmol-1
ΔHθ
f[H2O(l)] = -286kJmol-1
Using Hess’ Law; Route 1 = Route 2
7.4 HESS’S LAW
ΔHθ
f values give
the enthalpy
change going from
the element to the
compound](https://image.slidesharecdn.com/7-231014172504-adacbd37/85/7-4-Hess-s-Law-TE-ppt-5-320.jpg)
![SO2(g) + 2H2S(g)
3S(s) + 2H2(g) + O2(g)
3S(s) + 2H2O(l)
ΔHθ
r
Route 1
ΔHθ
f(SO2) +
2 x ΔHθ
f(H2S)
REACTANTS PRODUCTS
ELEMENTS
Step 3:
ΔHθ
f[SO2(g)] = -297 kJmol-1
ΔHθ
f[H2S(g)] = -20.2 kJmol-1
ΔHθ
f[H2O(l)] = -286kJmol-1
Using Hess’ Law; Route 1 = Route 2
7.4 HESS’S LAW
ΔHθ
f values give
the enthalpy
change going from
the element to the
compound
3 x ΔHθ
f(S) +
2 x ΔHθ
f(H2O)](https://image.slidesharecdn.com/7-231014172504-adacbd37/85/7-4-Hess-s-Law-TE-ppt-6-320.jpg)
![SO2(g) + 2H2S(g)
3S(s) + 2H2(g) + O2(g)
3S(s) + 2H2O(l)
ΔHθ
r
Route 1
-297 +
(2 x -20.2)
REACTANTS PRODUCTS
ELEMENTS
Step 3:
ΔHθ
f[SO2(g)] = -297 kJmol-1
ΔHθ
f[H2S(g)] = -20.2 kJmol-1
ΔHθ
f[H2O(l)] = -286kJmol-1
Using Hess’ Law; Route 1 = Route 2
7.4 HESS’S LAW
ΔHθ
f(s) is zero
because its an
element
(3 x 0) +
(2 x -286)](https://image.slidesharecdn.com/7-231014172504-adacbd37/85/7-4-Hess-s-Law-TE-ppt-7-320.jpg)