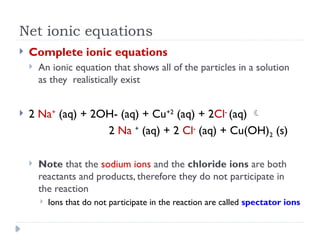
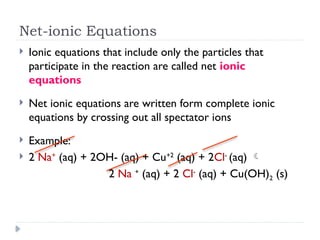
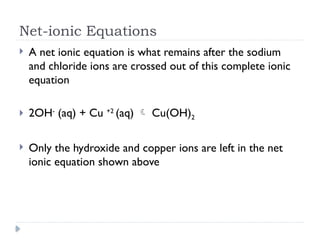
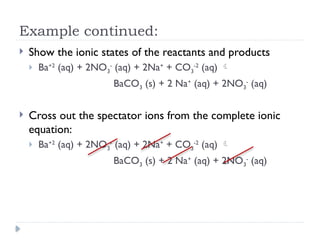
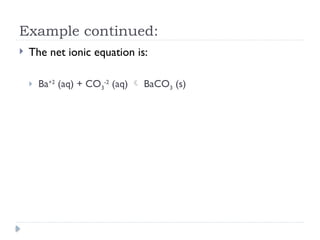
Net ionic equations demonstrate only the chemical species directly involved in a reaction, excluding spectator ions that do not participate. To create a net ionic equation, one must recognize ions, apply solubility rules, and remove spectator ions from the complete ionic equation. An example illustrates this process through the reaction between barium nitrate and sodium carbonate, resulting in the net ionic equation: Ba²⁺ (aq) + CO3²⁻ (aq) → BaCO3 (s).