Carbon dating works by measuring the ratio of carbon-14 to carbon-12 in organic material. Over time, the amount of carbon-14 decreases as it radioactively decays. By measuring the current ratio and knowing the half-life, the age of once-living material can be estimated. However, the production rate of carbon-14 in the atmosphere may change in the future due to factors like solar activity or human activity like nuclear weapons testing. This could affect the accuracy of carbon dating beyond about 50,000 years ago.





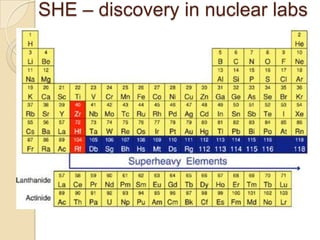
![Asymmetry term
Neutron and proton states with
Neutrons Protons same spacing .
Crosses represent initially
occupied states in ground state.
If three protons were turned into
neutrons the extra energy required
would be 3 3 .
In general if there are N Z
excess protons over neutrons the
extra energy is [(N Z)/2]2 .
relative to Z = N.
(N Z )2
E Asym aa
A 1/A](https://image.slidesharecdn.com/10-nuclearphysics-121103223226-phpapp01/85/nuclear-physics-11-320.jpg)