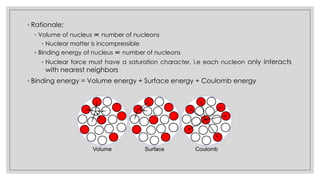
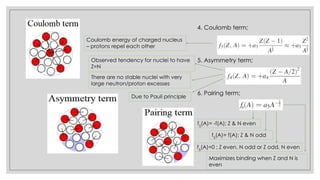
This document discusses the liquid drop model of nuclear structure. It describes the liquid drop model as treating the nucleus like a drop of incompressible liquid with short-range nuclear forces between nucleons. The liquid drop model uses a semi-empirical mass formula to predict nuclear binding energies based on volume, surface tension, Coulomb repulsion, and other terms. It provides coefficients that were fitted to experimental nuclear binding energy data and can estimate energies for many stable and unstable nuclei.