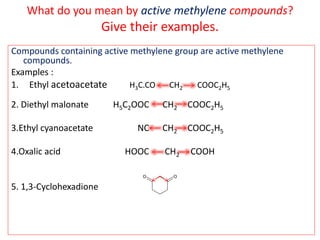
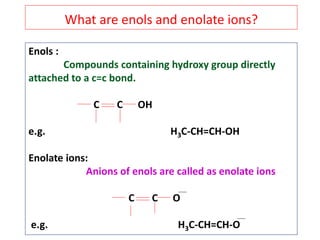
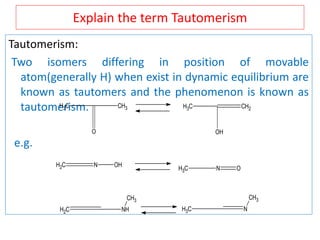
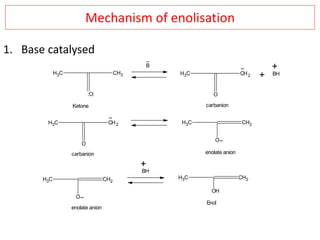
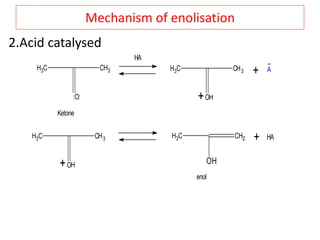
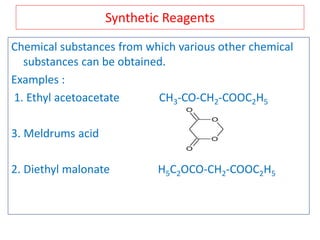
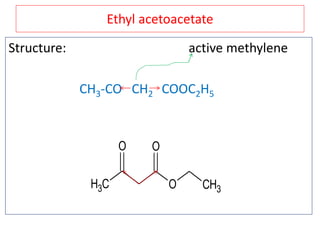
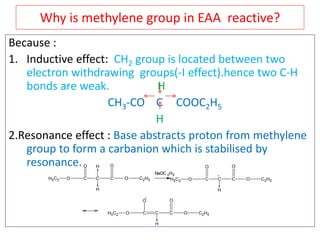
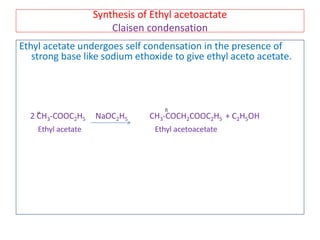
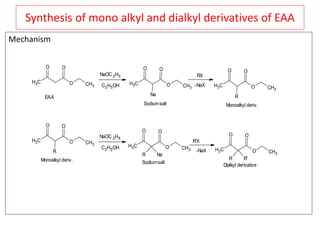
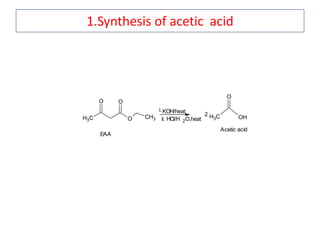
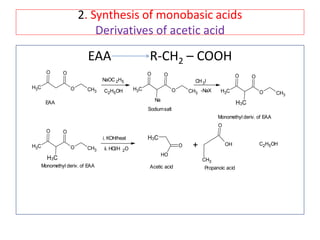
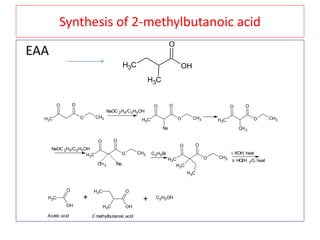
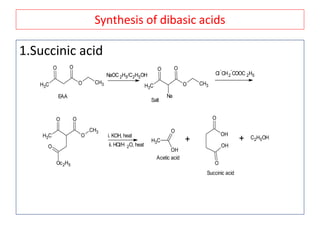
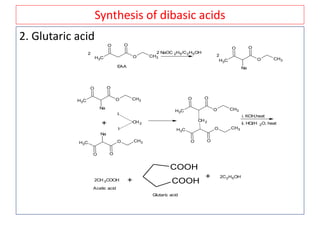
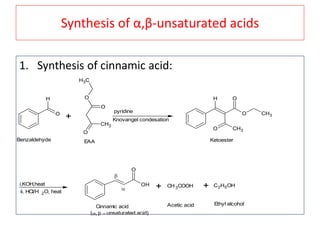
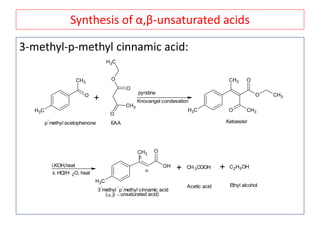
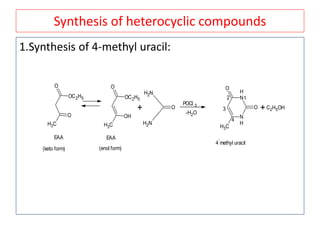
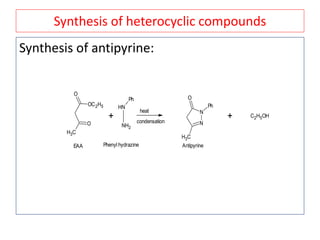
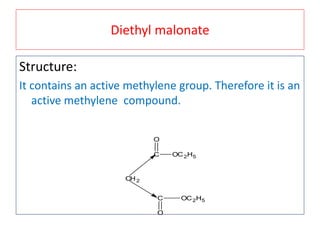
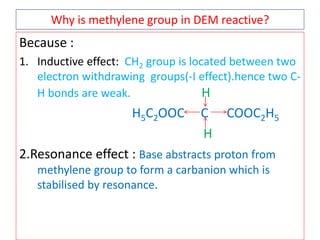
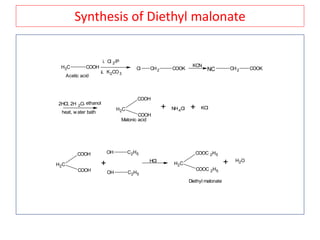
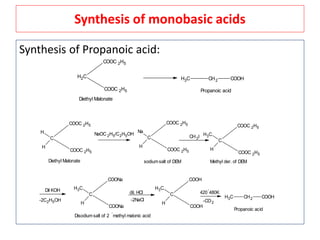
The document outlines a comprehensive study on enolates and their synthetic applications, focusing on active methylene compounds, enols, enolate ions, and their mechanisms. Key topics include the synthesis of ethyl acetoacetate and diethyl malonate through various methods like Claisen condensation, along with alkylation processes and hydrolysis for producing carboxylic acids. It also discusses tautomerism and provides examples of synthetic applications of both compounds in producing various organic chemicals.