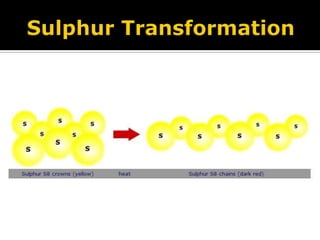
Plastic sulfur is formed when molten sulfur is rapidly cooled in water. It has a rubber-like structure with sulfur chains aligned parallel to one another and held together by van der Waals forces. This plastic sulfur is unstable at room temperature as the sulfur chains slowly rearrange into crystalline structures, making the material more brittle over time. Sulfur exists in different states depending on temperature, being solid below 95.6°C, liquid from 113-445°C, and transforming from low to high viscosity as it is heated through this range.