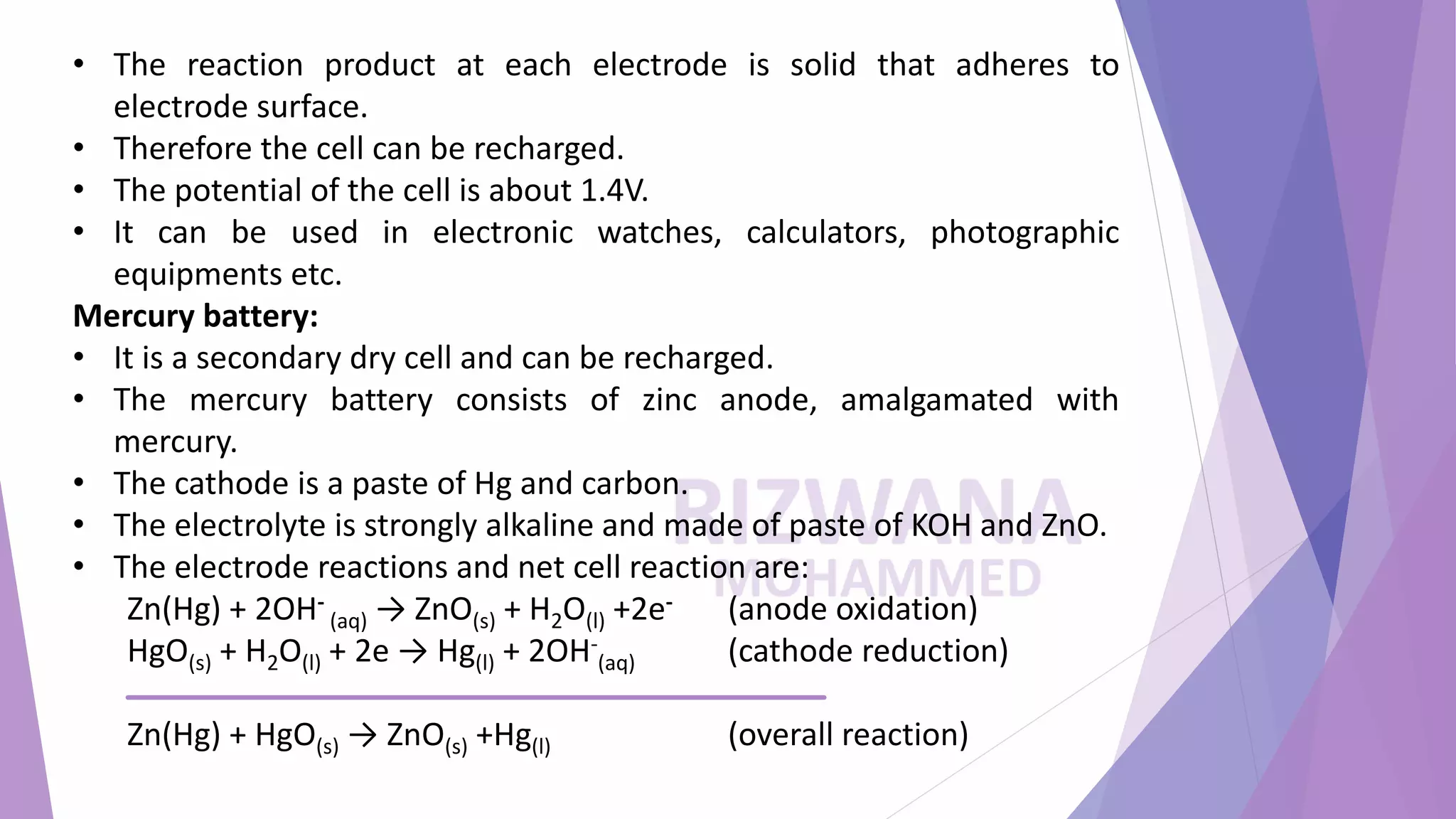
The document summarizes three types of rechargeable batteries: lead-acid, nickel-cadmium, and mercury batteries. It describes the electrode reactions, electrolytes, and applications of each battery type. Lead-acid batteries use lead and lead dioxide electrodes with sulfuric acid electrolyte and are used in cars and inverters. Nickel-cadmium batteries have cadmium and nickel oxide electrodes with an alkaline electrolyte and are used in small electronics. Mercury batteries contain zinc, mercury, and carbon electrodes with a paste electrolyte and provide a constant voltage for hearing aids and watches.