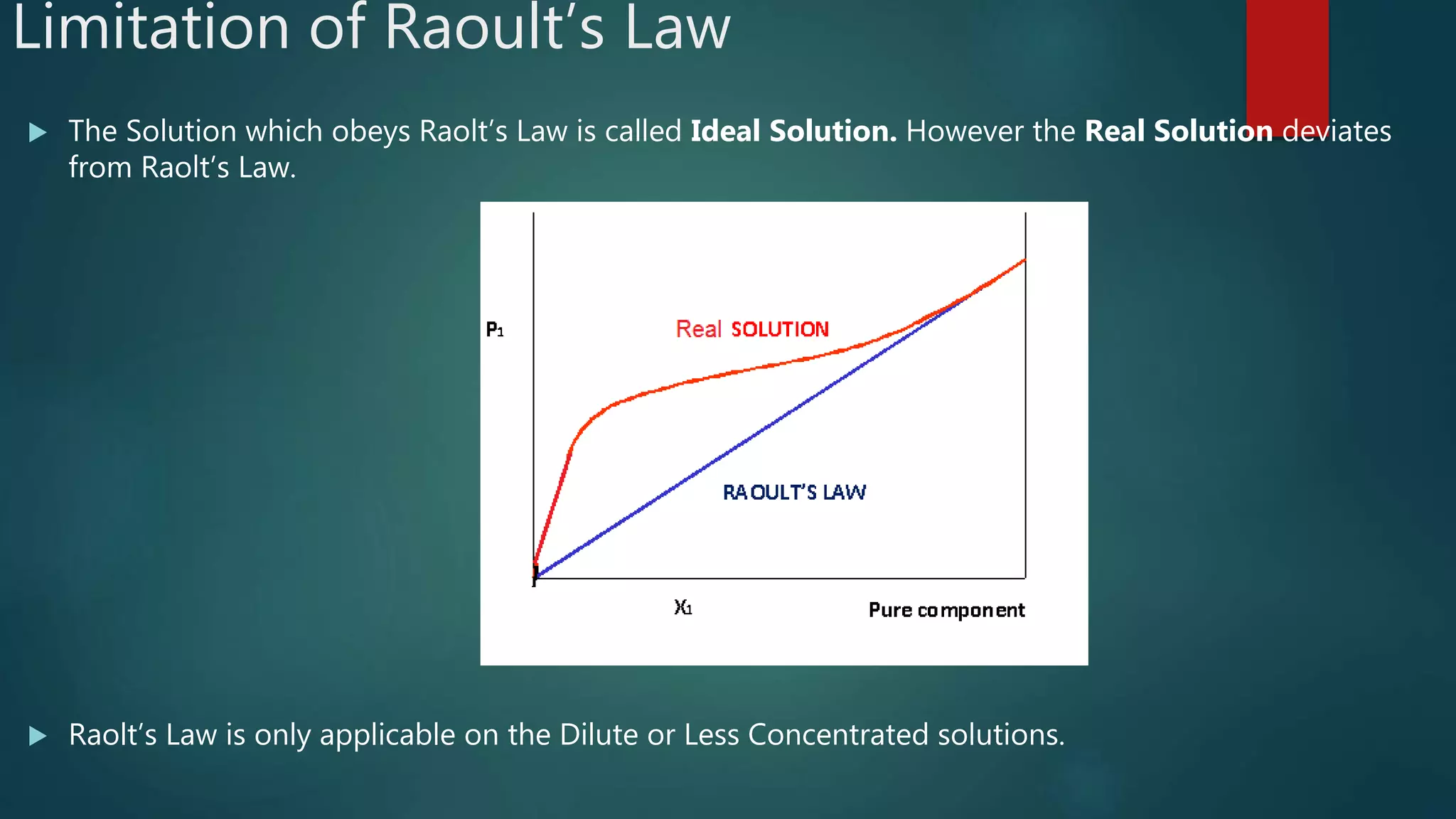
Raoult's Law states that the vapor pressure of a solution is directly proportional to the mole fraction of solvent. It describes how solute particles affect the vapor pressure, boiling point, and freezing point of a solution, including key concepts like colligative properties and limitations regarding ideal solutions. The document outlines various properties such as lowering of vapor pressure, boiling point elevation, and freezing point depression, with formulas and examples illustrating these phenomena.