Embed presentation
Download to read offline



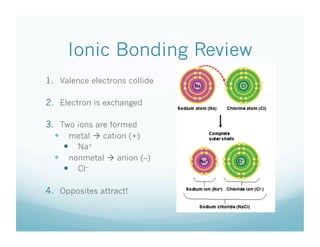
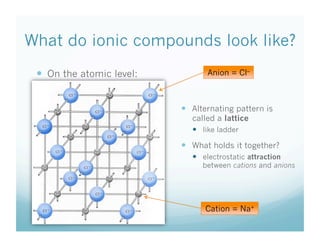
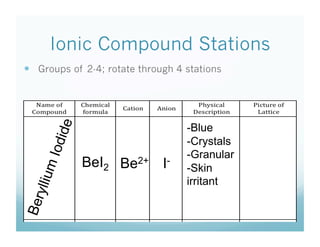

The document discusses the structure of ionic compounds. It begins by explaining that ionic compounds appear as white crystals to the naked eye and have an alternating lattice structure of cations and anions on the atomic level. The ions are held together by electrostatic attraction. Students are then told they will do a lab rotating through stations on different ionic compounds.






