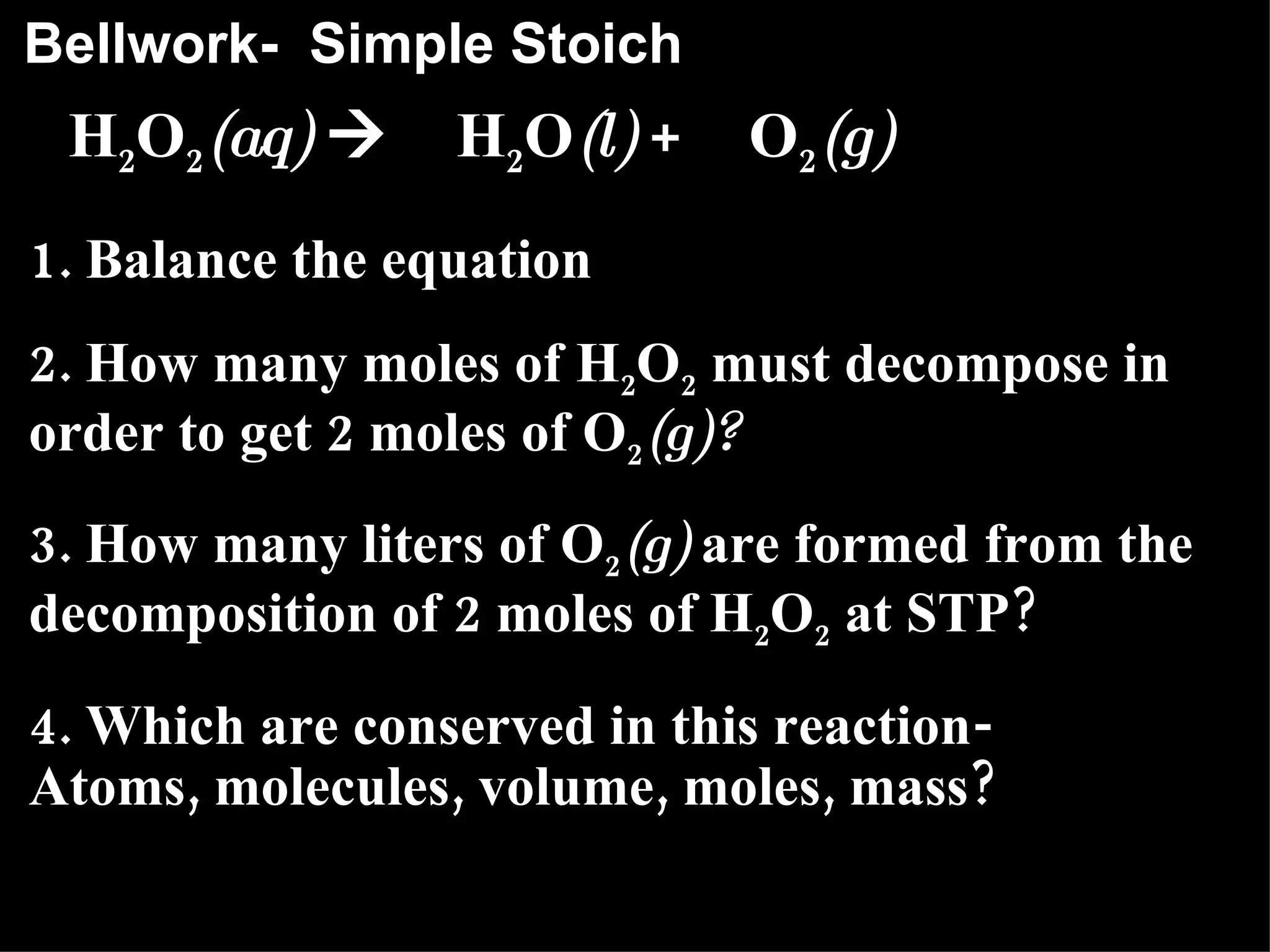
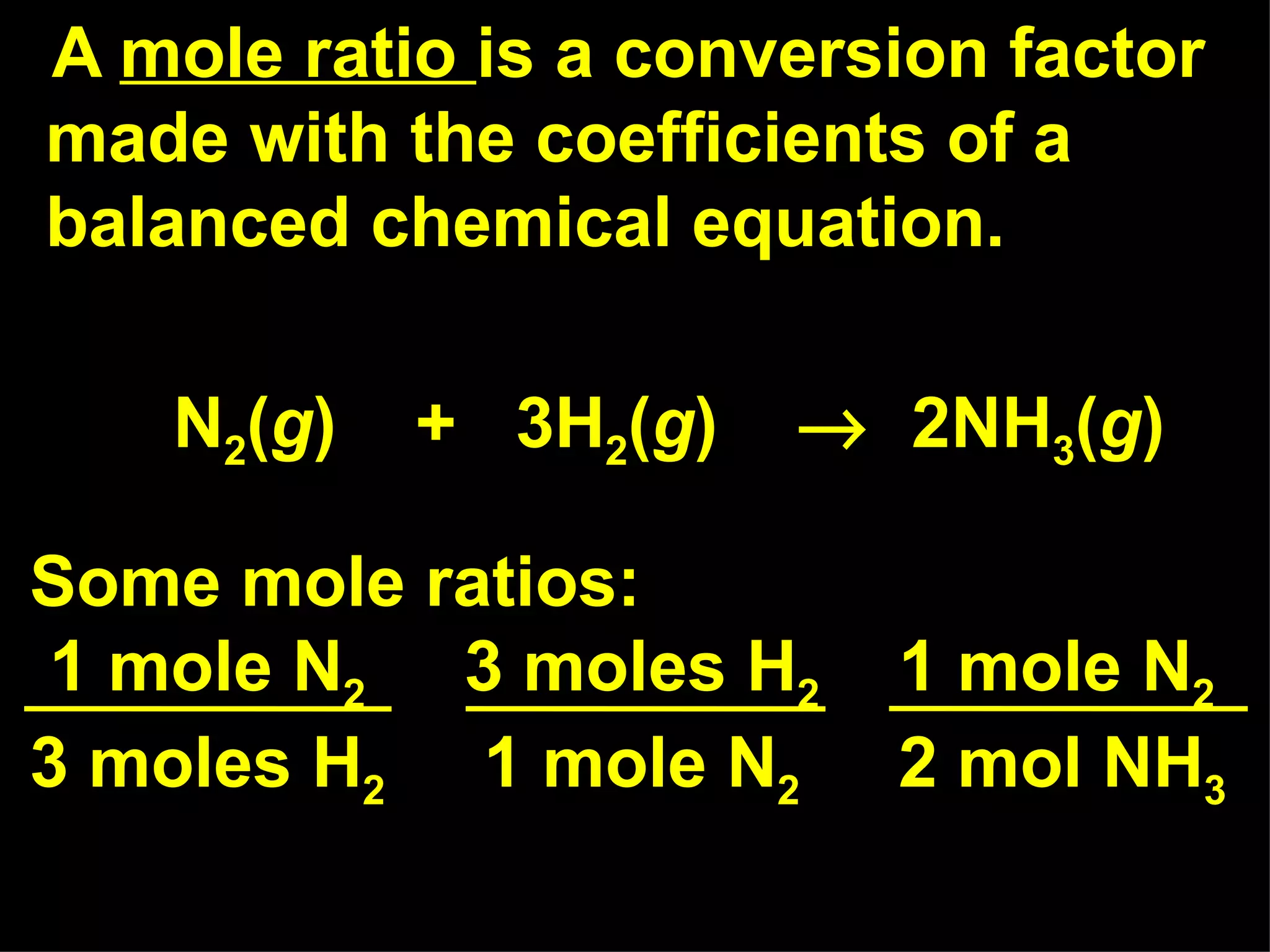
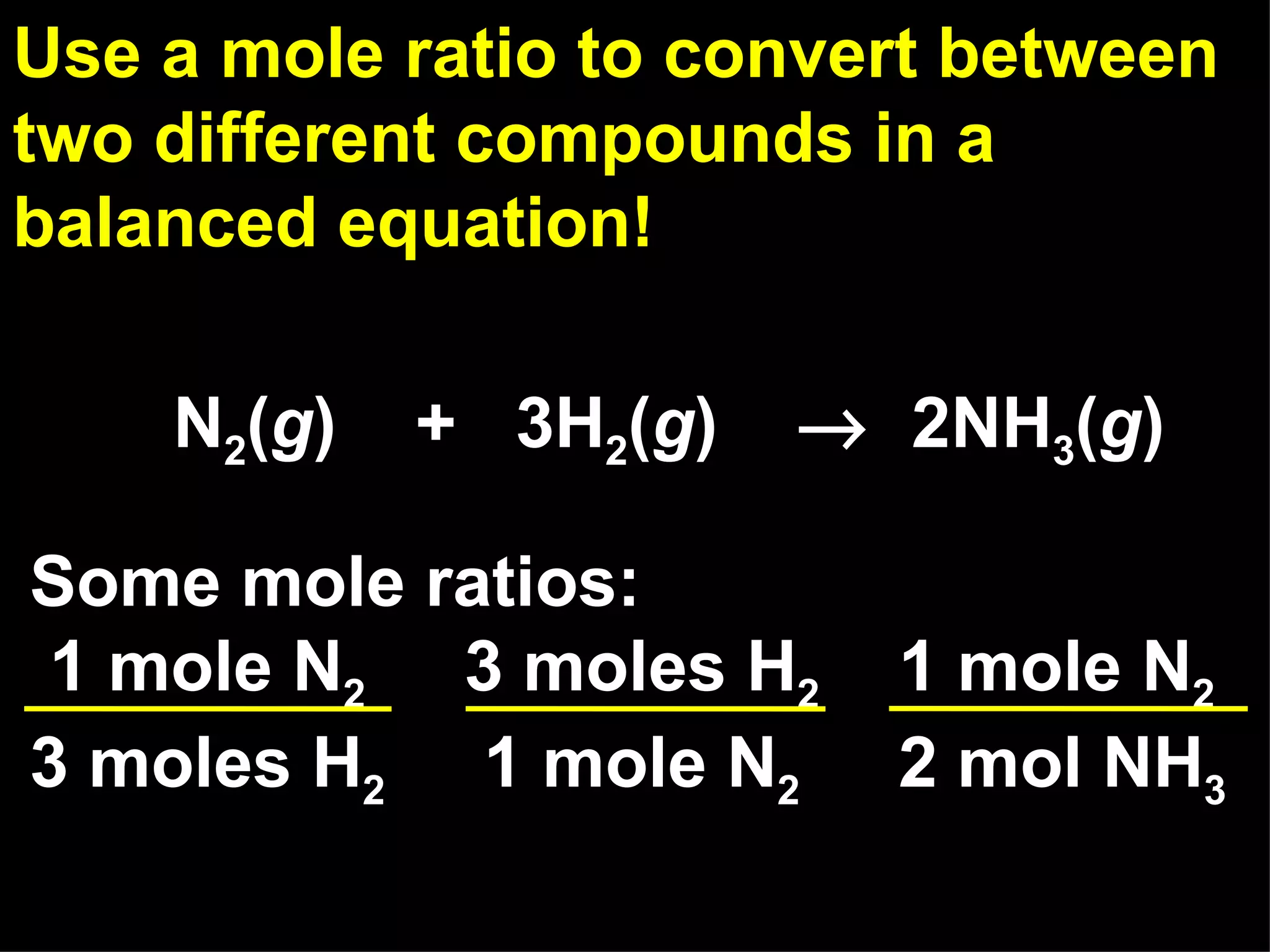
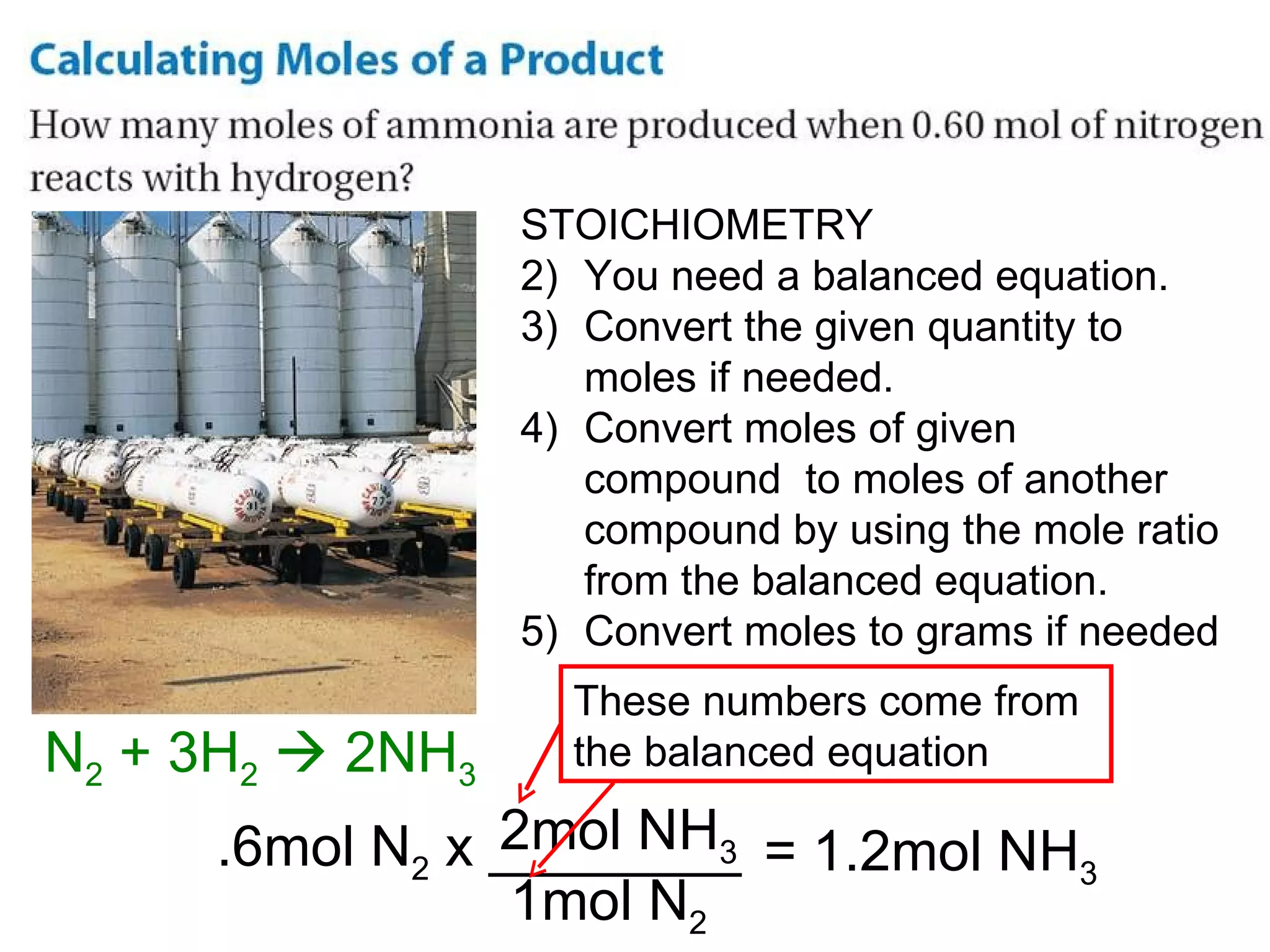
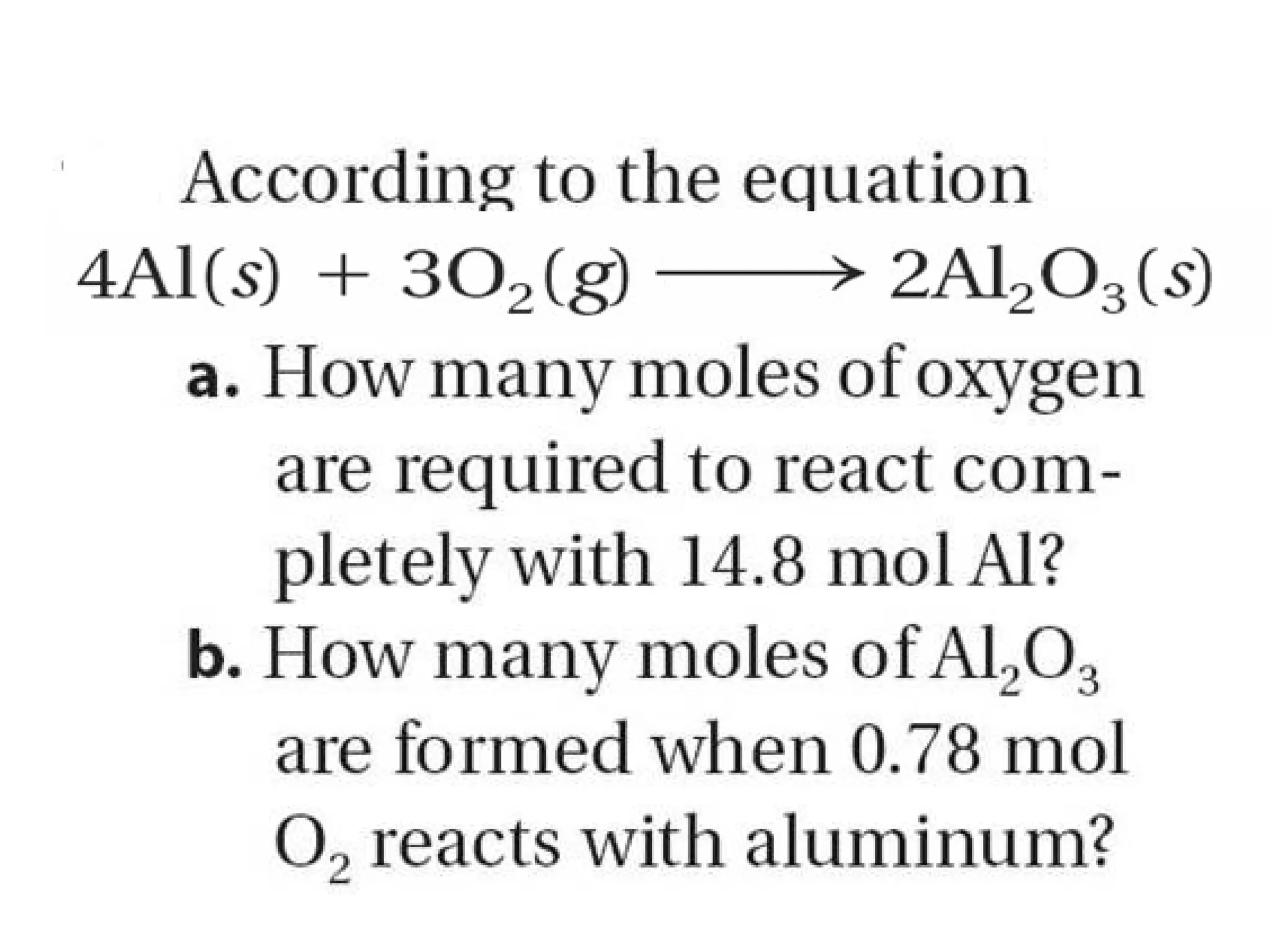
The document provides an explanation of stoichiometry and how to use mole ratios to solve stoichiometry problems. It defines mole ratios as conversion factors made from the coefficients of a balanced chemical equation. It outlines the steps to solve stoichiometry problems: 1) use a balanced equation, 2) convert quantities to moles if needed, 3) use mole ratios to convert between compounds, and 4) convert back to other units like grams if needed. An example problem is shown calculating moles of products from moles of reactants.