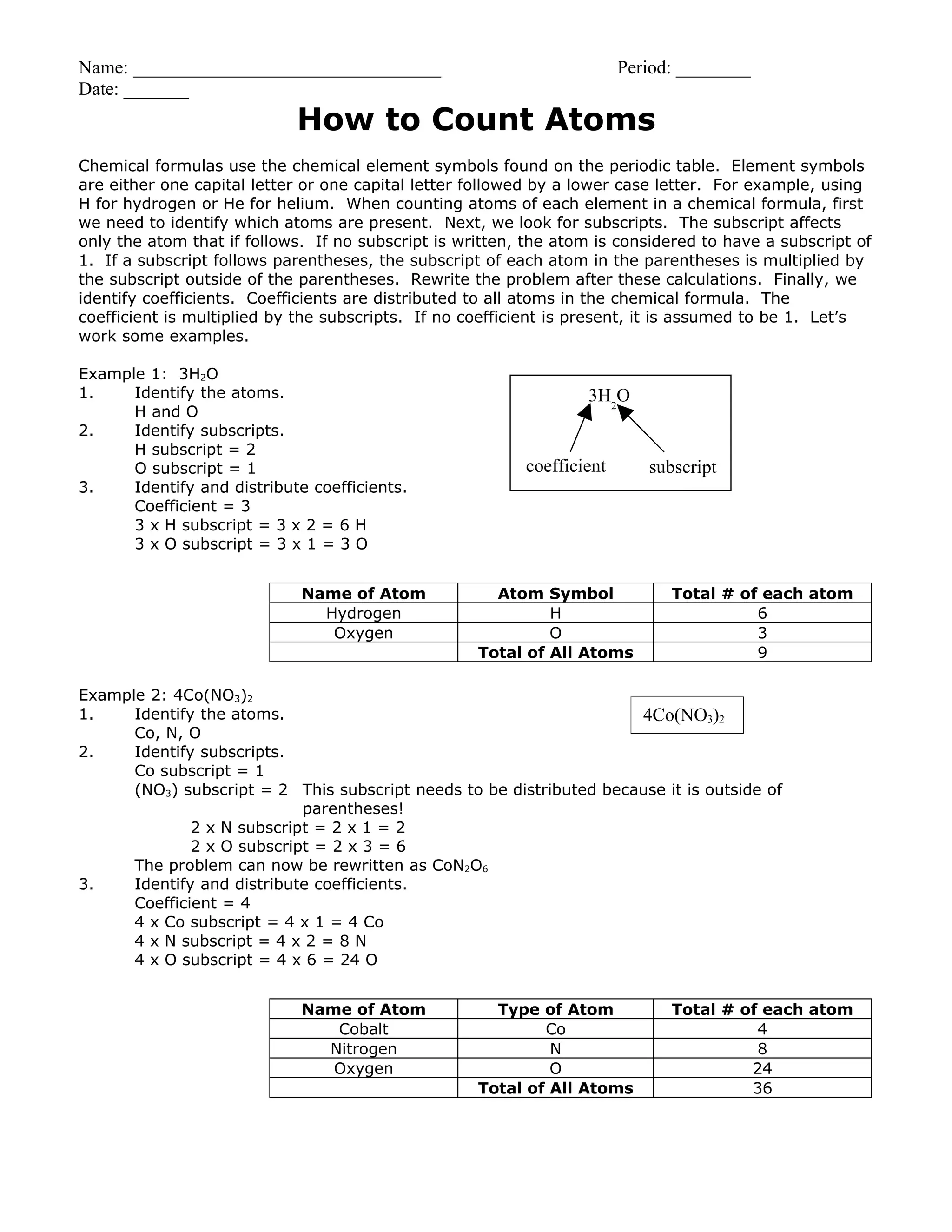
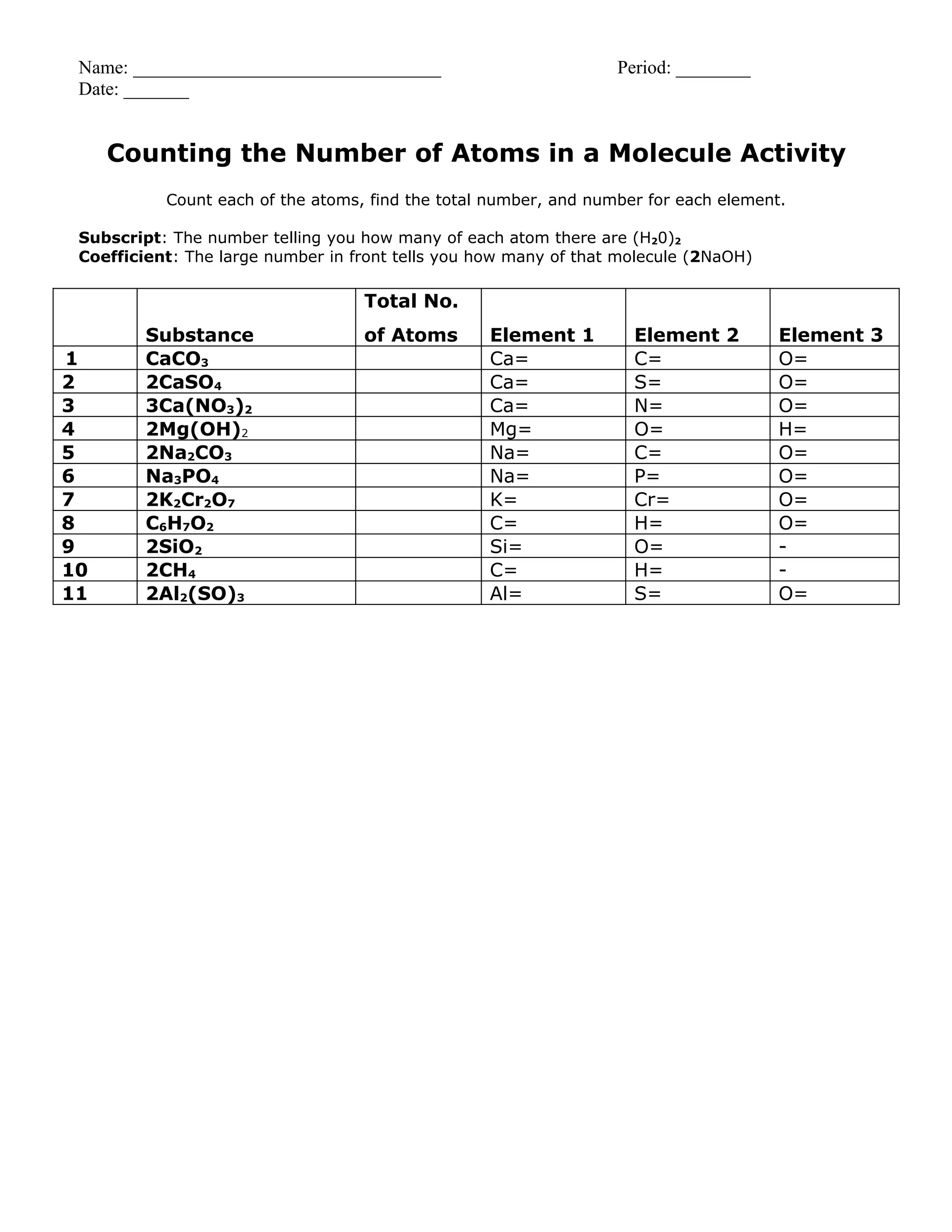
This document provides instructions for counting atoms in chemical formulas. It explains that chemical formulas use element symbols from the periodic table, which may include subscripts to indicate the number of each atom. It notes that if no subscript is present, the atom count is 1, and if a subscript is outside parentheses it is distributed to each atom inside. The document also explains that coefficients represent the total number of that chemical formula and are distributed by multiplying the coefficient by each atom's subscript. It provides two examples working through identifying atoms, subscripts, and distributing coefficients to arrive at the total count of each element.