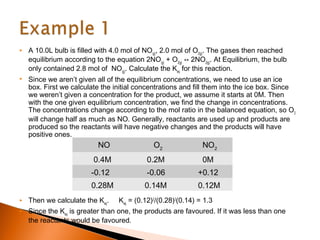
The document explains the concept of an ICE (Initial, Change, Equilibrium) box used for calculating equilibrium concentrations in chemical reactions. It details how to set up the ICE box, derive equilibrium concentrations, and calculate the equilibrium constant (K_eq) with examples. Additionally, it discusses applications of ICE boxes for determining ionization constants of weak acids and bases.