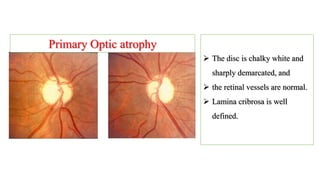
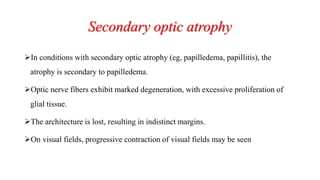
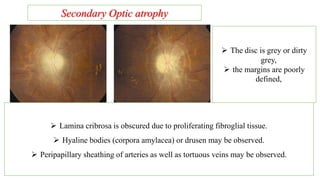
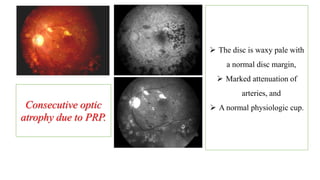
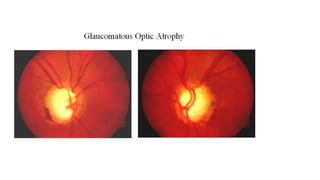
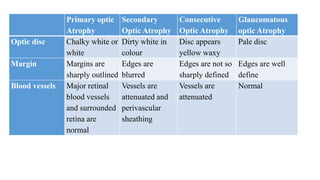
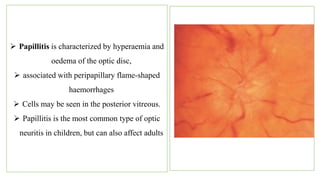
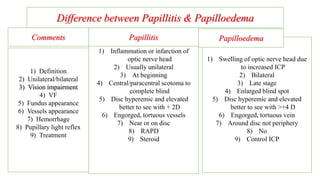
The document discusses various aspects of the optic nerve, including its anatomy, diseases, and types of optic atrophy such as primary, secondary, consecutive, and glaucomatous optic atrophy. It elaborates on conditions like optic neuritis, detailing differences between papillitis and papilloedema, as well as toxic and nutritional optic neuropathies. Treatment strategies focus on early diagnosis and cessation of harmful substances, emphasizing the importance of nutritional supplementation and interventions for improvements in vision.






















![Clinical Features
Usually, there is a sudden or rapid painless bilateral vision loss.
Simultaneous involvement of both eyes is more common with nutritional
deficiency, toxic and some hereditary disorders,
but monocular onset and fellow eye involvement occurring later (days, weeks or
months) is more common with Leber hereditary optic neuropathy.
Visual loss occurs, ranging from mild [6/7.5 (20/25)] to severe (finger-counting).](https://image.slidesharecdn.com/03lectureneuro-210421060648/85/03-lecture-neuro-31-320.jpg)