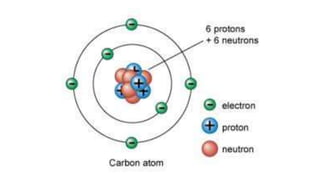
This document provides an overview of the development of atomic theory from ancient Greek philosophers to modern atomic structure. It summarizes key contributors and discoveries:
- Democritus proposed atoms as indivisible particles (5th century BC). John Dalton's atomic theory (1803) stated all matter is made of atoms that cannot be created, destroyed, or divided.
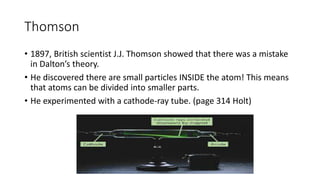
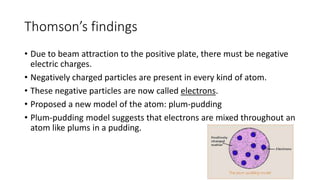
- J.J. Thomson's discovery of the electron (1897) showed atoms can be divided. Ernest Rutherford's gold foil experiment (1909) demonstrated atoms are mostly empty space with a dense nucleus.
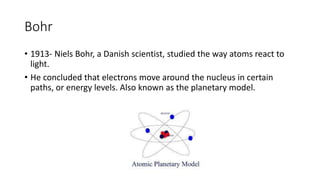
- Niels Bohr's model (1913) showed electrons orbiting the nucleus in defined energy levels. Modern atomic theory describes electrons in