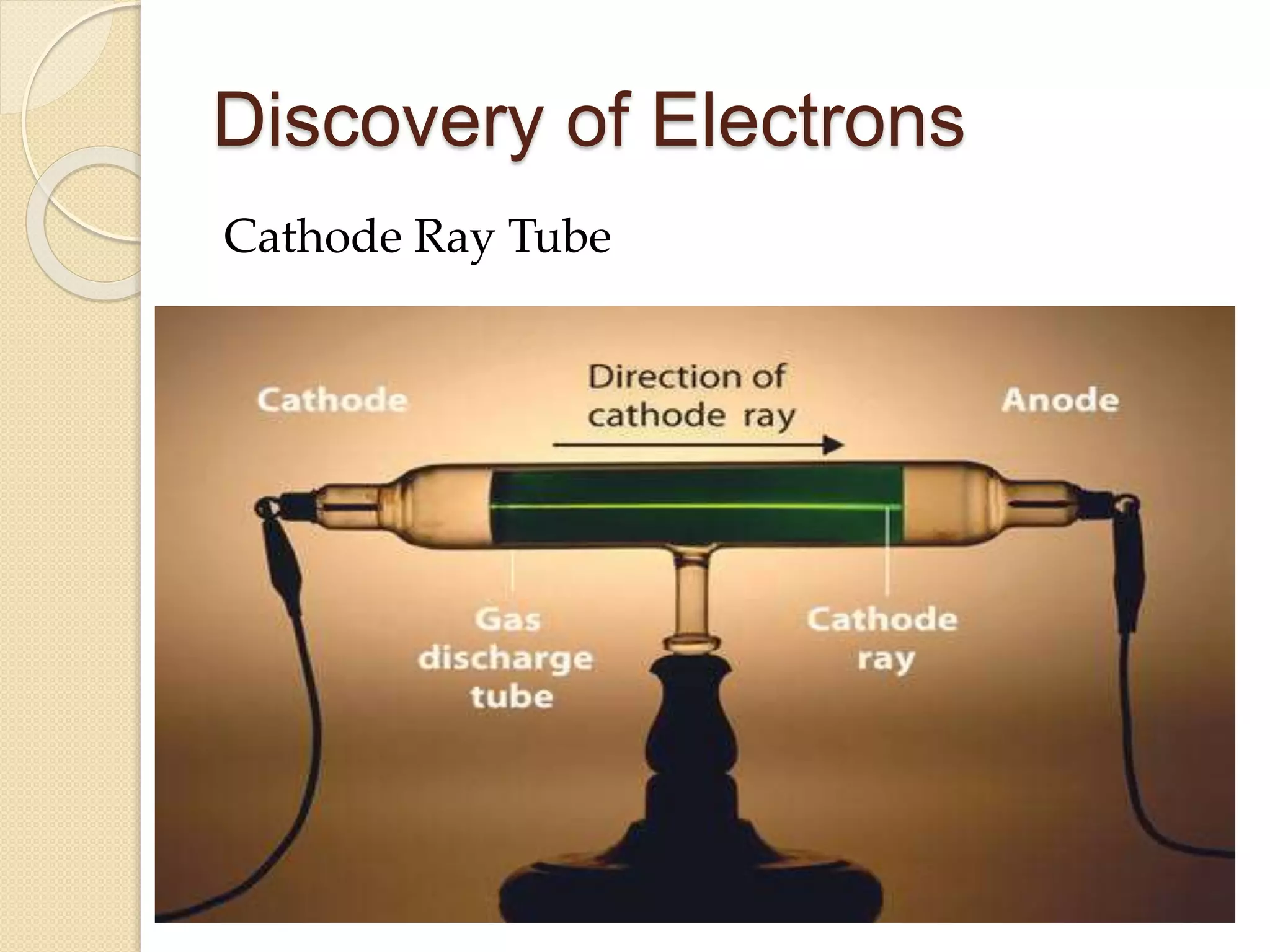
The document outlines key contributions to the discovery of electrons and protons by notable scientists including Sir William Crookes, J.J. Thomson, Eugen Goldstein, and Ernest Rutherford. It highlights Thomson's identification of electrons through cathode ray experiments and Rutherford's gold foil experiment which revealed the existence of protons and the atomic nucleus. The findings emphasize the nature of atomic structure and the behavior of subatomic particles under electric fields.