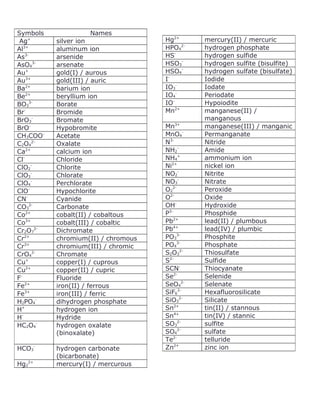
This document lists common chemical symbols and names for ions and ionic compounds. It provides the symbols and two common name formats for many monoatomic, polyatomic, and transition metal ions. Examples include Ag+ for the silver ion, also called the silver(I) or aurous ion, and HPO42- for the hydrogen phosphate ion. In total, over 50 different ions and ionic compounds are defined in the table.