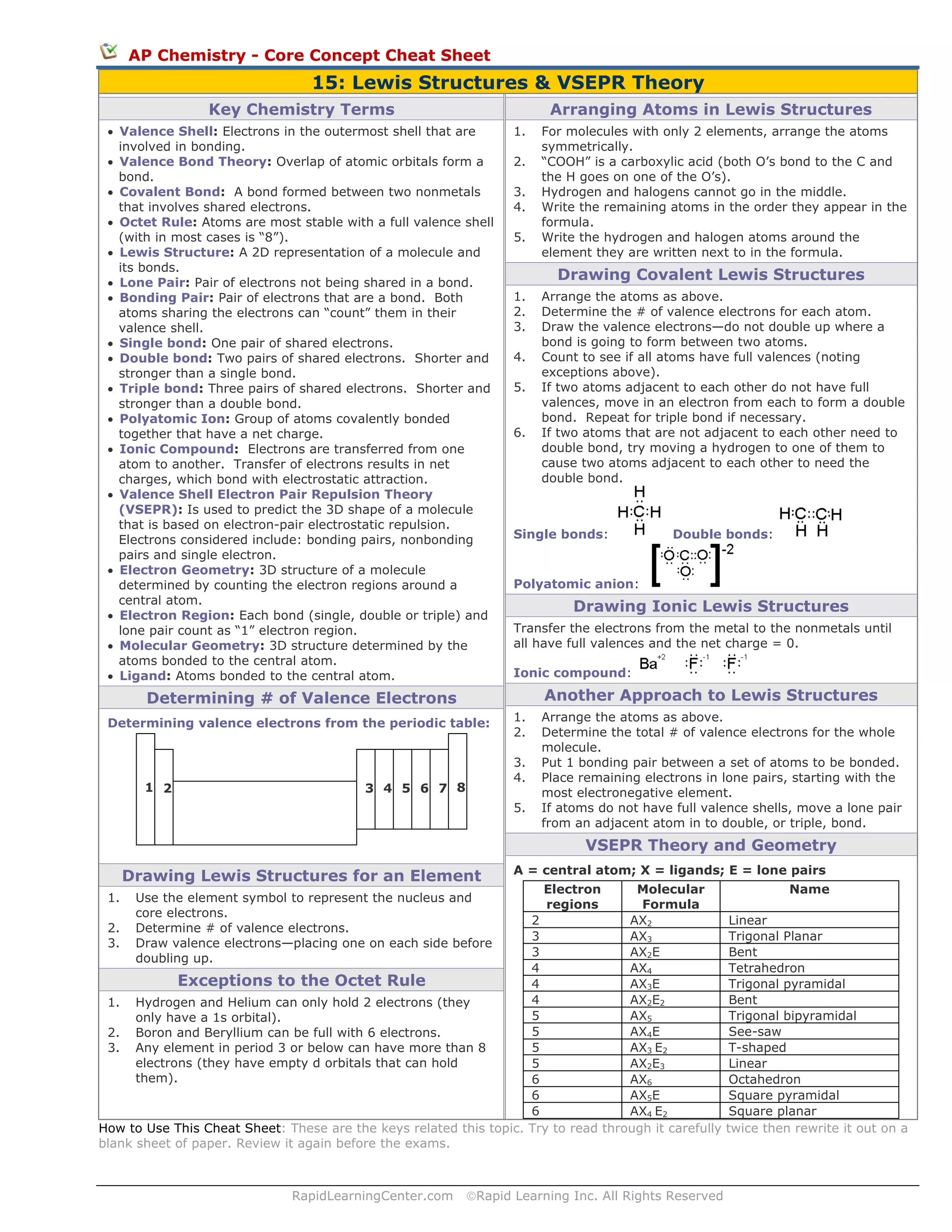
1. The document provides an overview of key concepts for Lewis structures and VSEPR theory, including how to draw Lewis structures for covalent compounds and ions.
2. It explains valence shell electron pair repulsion theory (VSEPR) which is used to predict the 3D shape of molecules based on electron pairs around a central atom.
3. A table is included that lists common molecular geometries determined by VSEPR theory based on the number of electron regions around the central atom.