Embed presentation
Downloaded 155 times


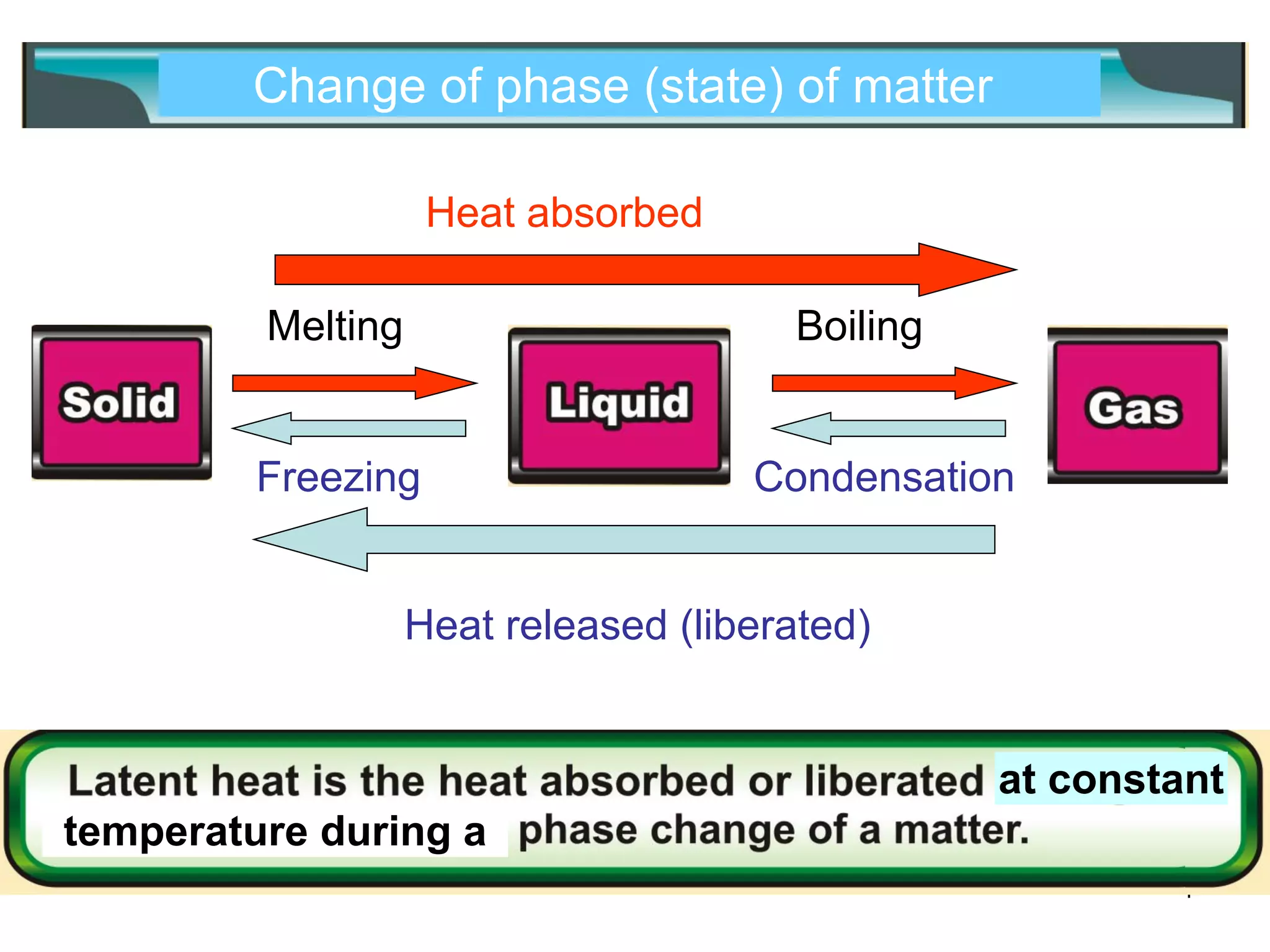
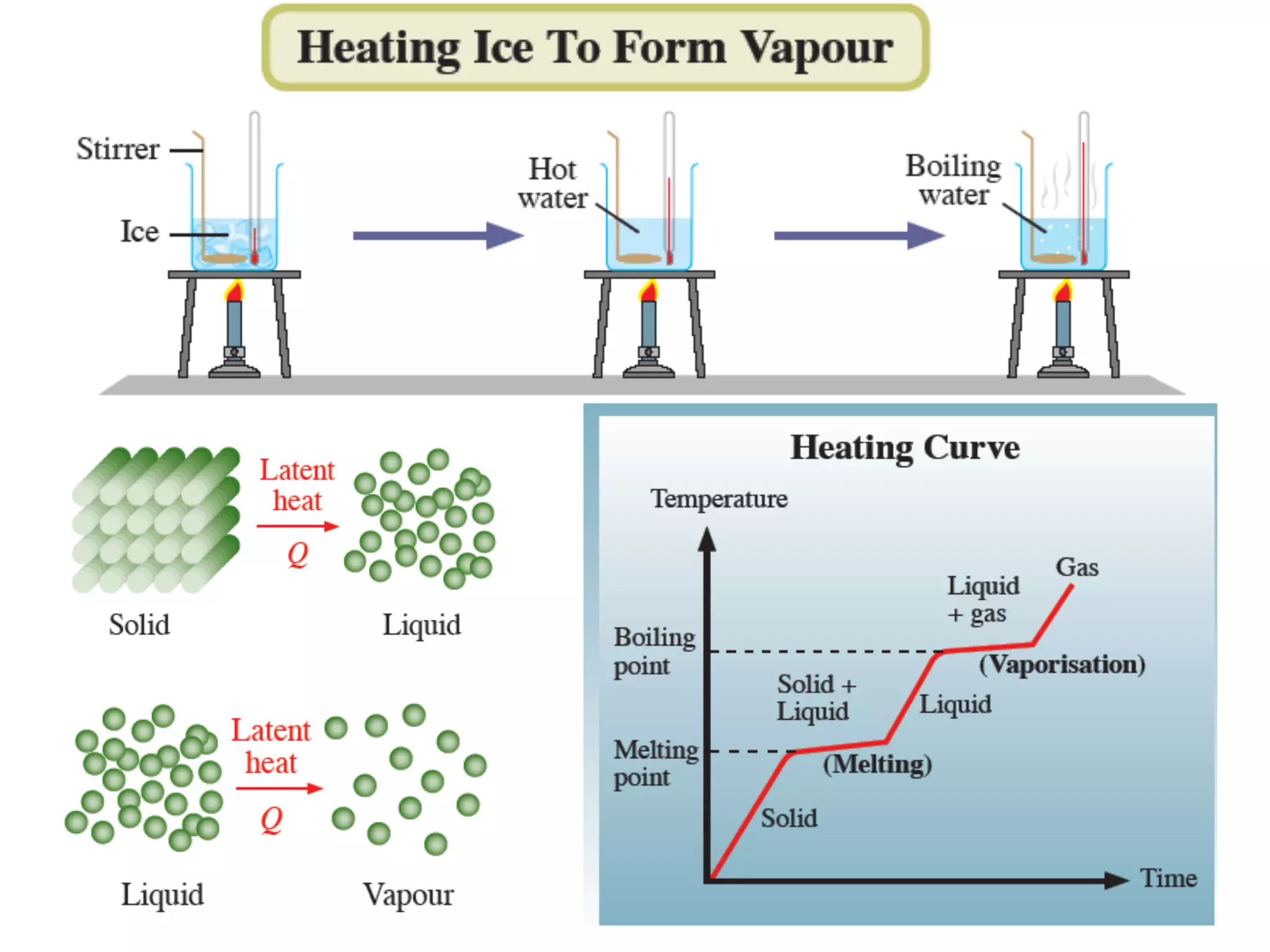

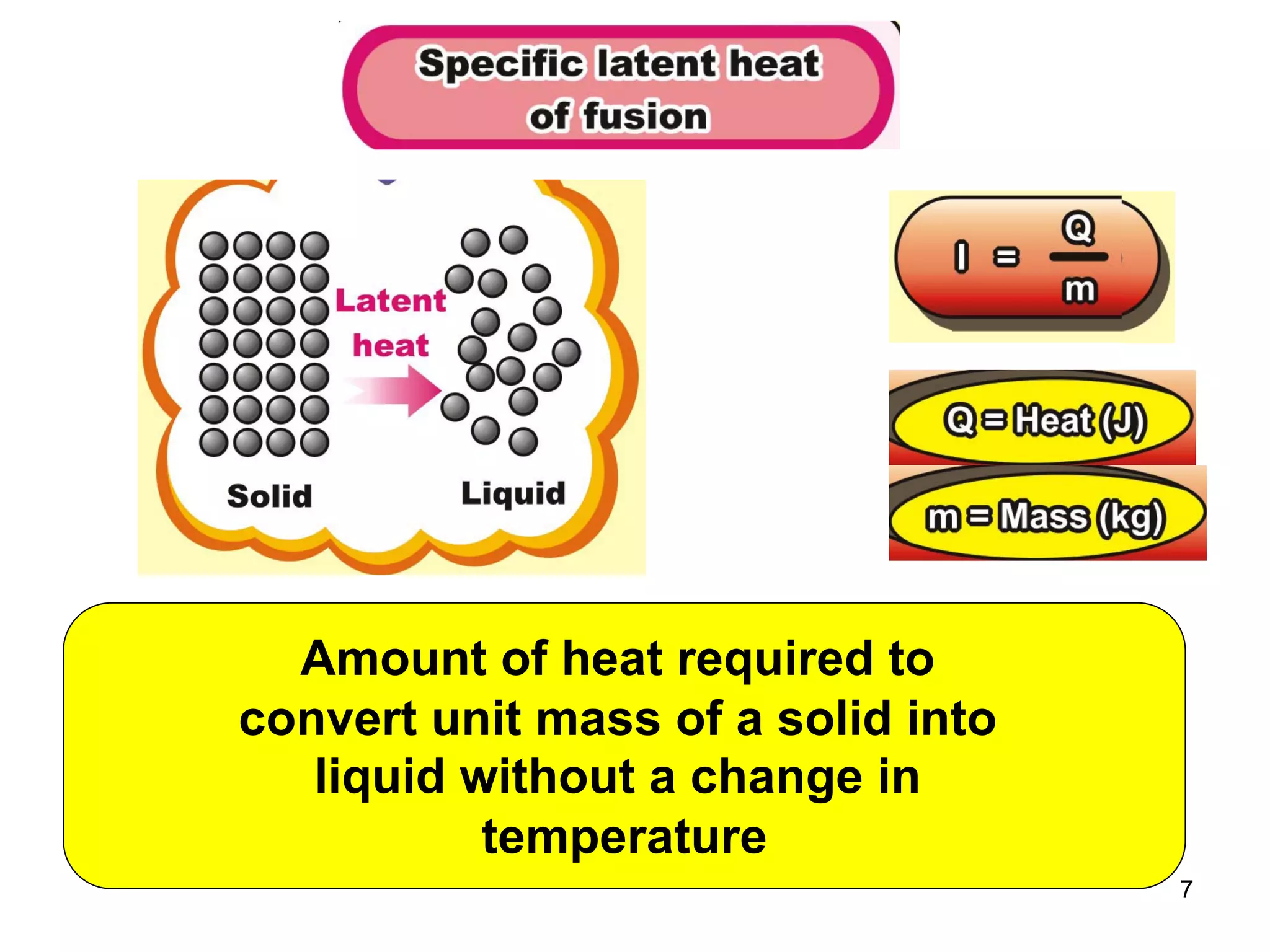

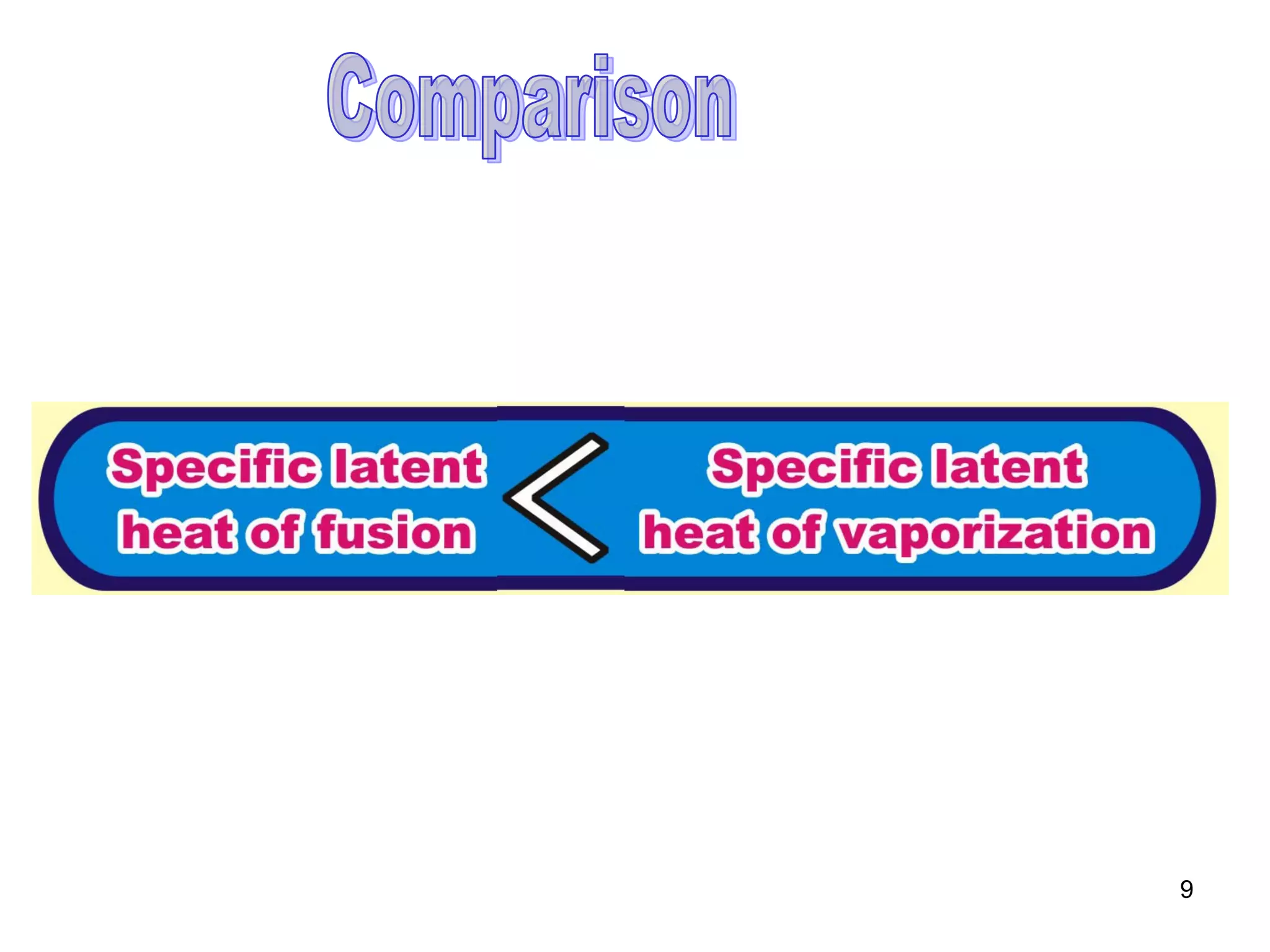
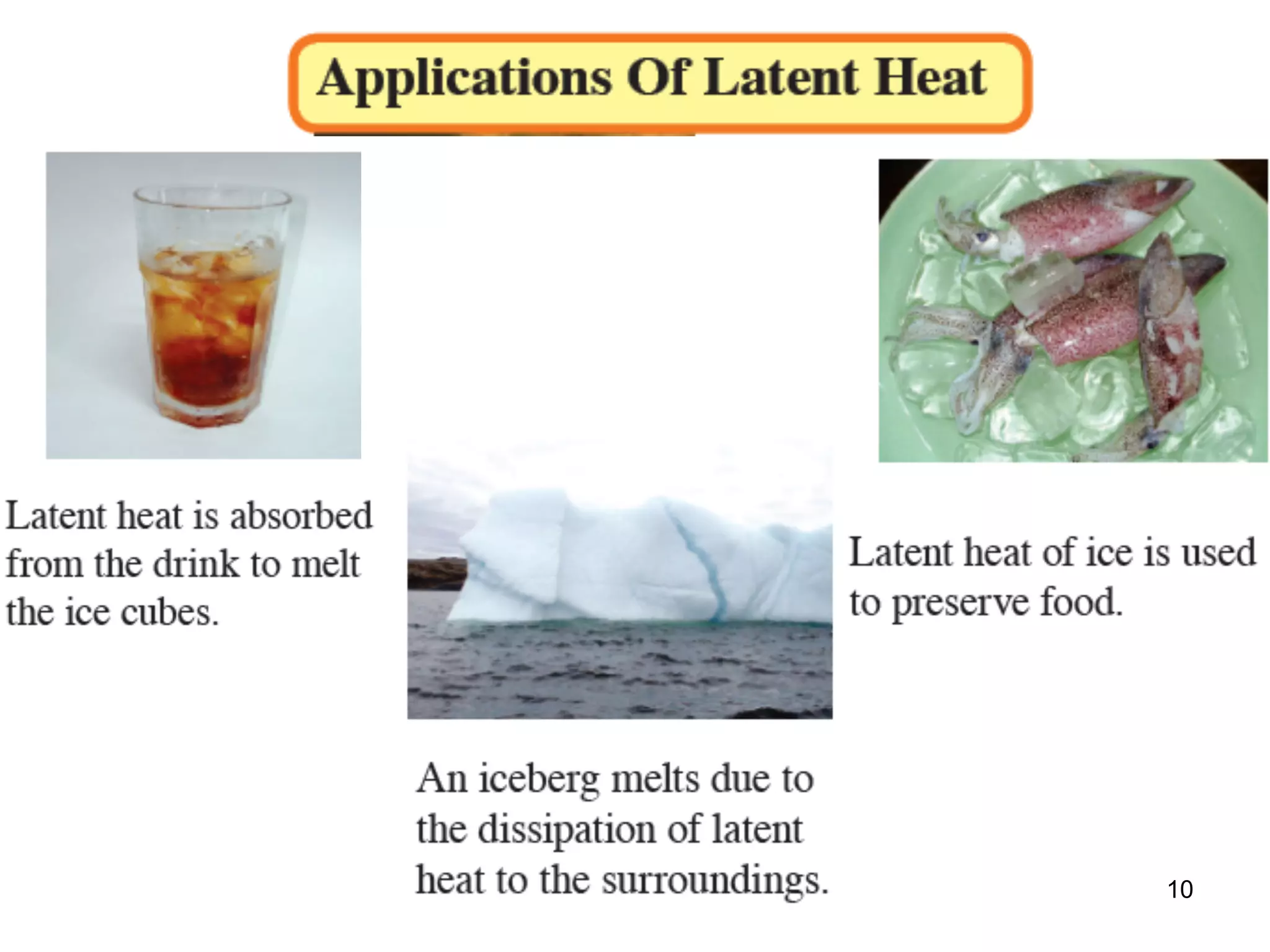
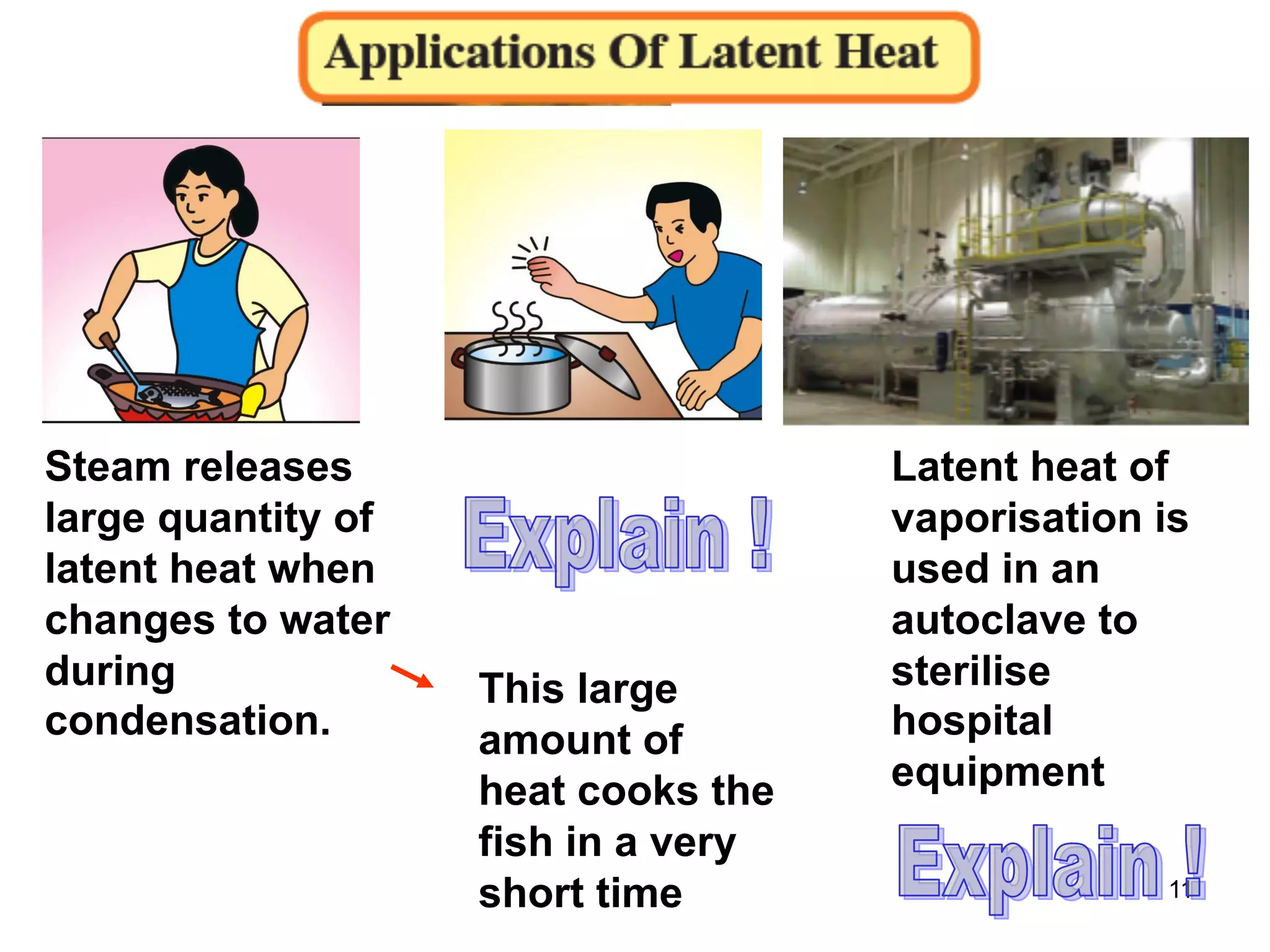
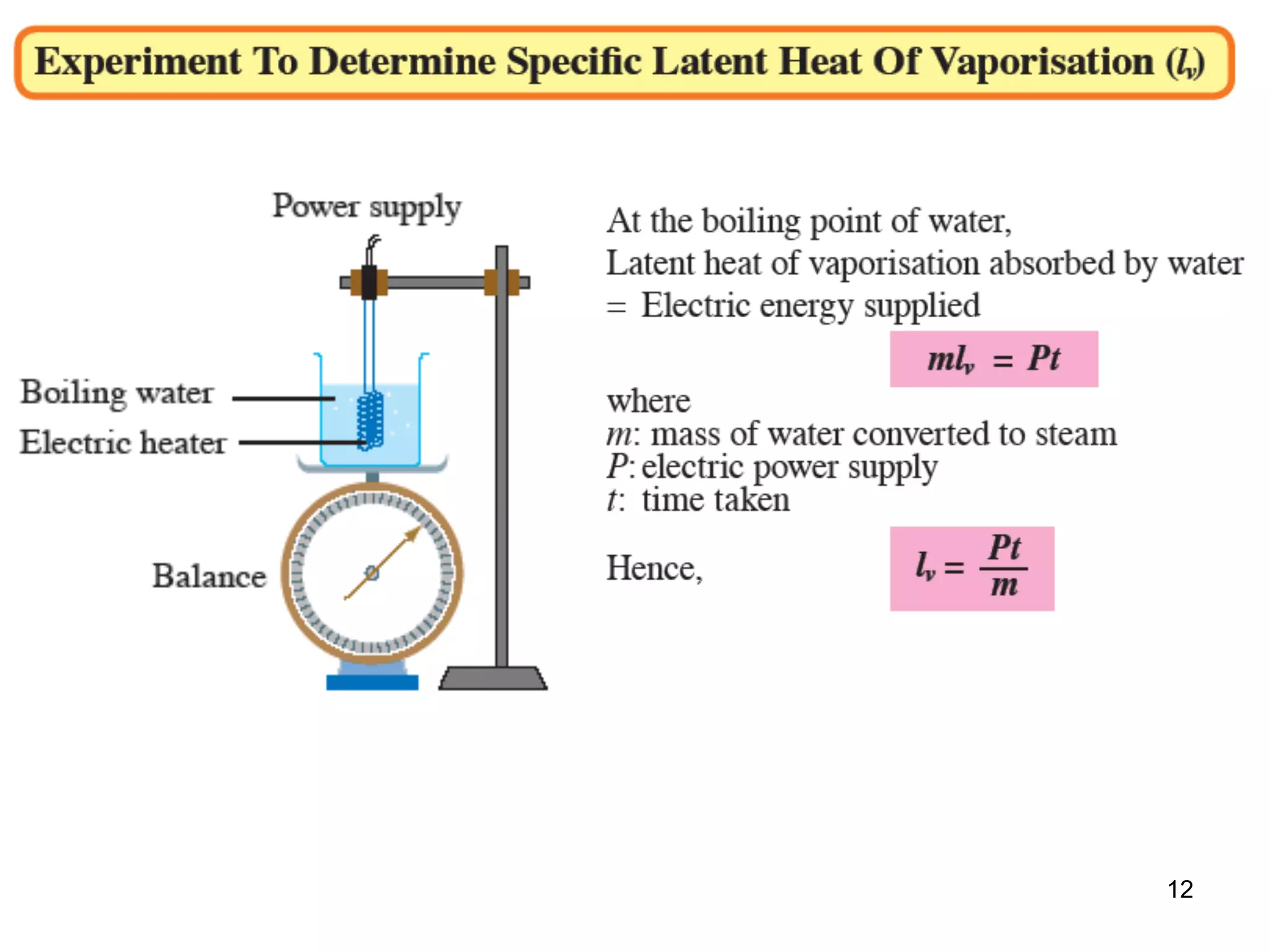


During a change of state, such as melting or boiling, heat is absorbed or released without a change in temperature. Specific latent heat is defined as the amount of heat required to change the state of one unit of mass of a substance. The specific latent heat of fusion is the amount of heat required to melt one unit of mass of a solid into a liquid, while the specific latent heat of vaporization is the amount of heat required to vaporize one unit of mass of a liquid into a gas. Latent heat is used in applications like autoclaving hospital equipment and cooking fish quickly using steam.












