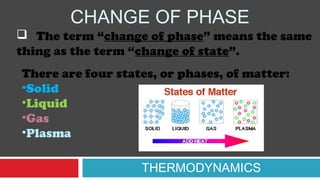
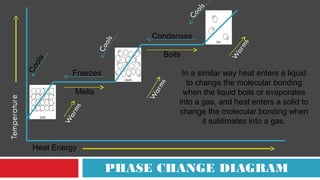
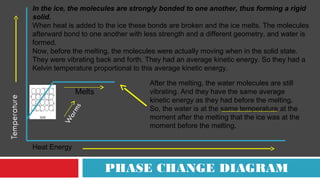
This document discusses phase changes and latent heat. It explains that during phase changes, heat is absorbed or released but temperature remains constant. The heat energy during phase changes is used to change the bonding between molecules rather than their kinetic energy. As a result, temperature stays the same even as heat is added or removed. The document provides examples of different phase changes like melting, freezing, evaporation and condensation. It also defines latent heat as the "hidden" heat absorbed or released during phase changes without changing temperature. Specific values of latent heat are given for various substances.

























![3. How much heat does a refrigerator need to remove
from 1.5 kg of water at 20.0 °C to make ice at 0°C?
[Hint: find heat removed for water at 20.0°C to water at 0°C, then find latent heat for
water at 0°C to ice at 0°C, and add the t]
Q total = mcΔ T + ml
EXAMPLE PROBLEMS](https://image.slidesharecdn.com/thermoreport-140928222834-phpapp01/85/Change-of-Phase-and-Latent-Heat-30-320.jpg)






![The specific latent heat (L) of a material …
is a measure of the heat energy (Q) per mass (m) released
or absorbed during a phase change.
is defined through the formula Q = mL.
is often just called the "latent heat" of the material.
uses the SI unit joule per kilogram [J/kg].
There are three basic types of latent heat each associated with
a different pair of phases.](https://image.slidesharecdn.com/thermoreport-140928222834-phpapp01/85/Change-of-Phase-and-Latent-Heat-37-320.jpg)



