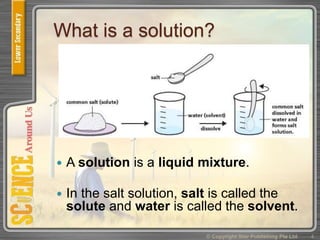
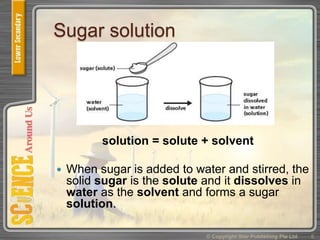
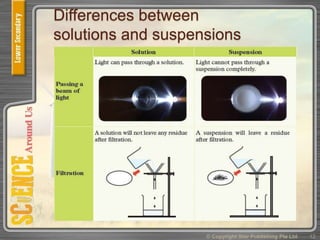
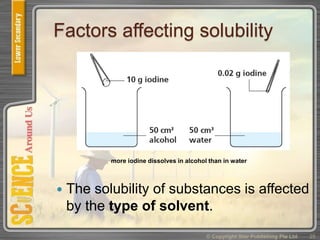
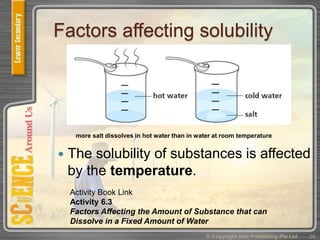
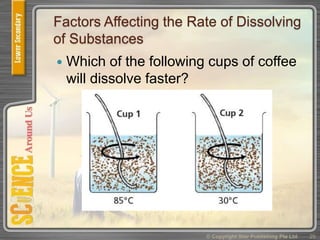
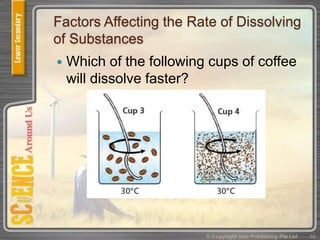
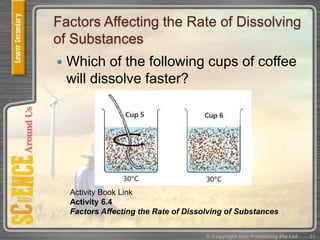
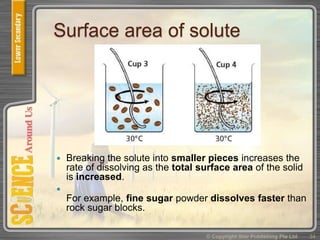
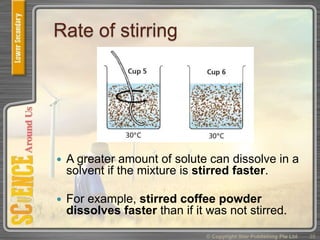
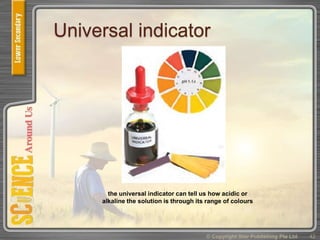
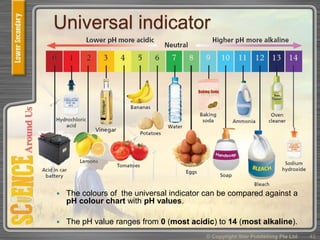
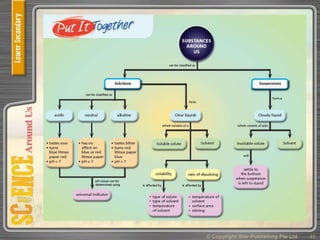
This document provides an overview of solutions, suspensions, and acids and bases. It defines solutions and suspensions, explaining that solutions are homogeneous mixtures while suspensions are heterogeneous. Key factors that affect solubility and dissolving rates are described, including temperature, surface area, and stirring. The document also introduces the pH scale and uses of indicators like litmus paper and universal indicator to determine if a solution is acidic, basic, or neutral.