Forced Degradation Study
•
4 likes•1,545 views
An Experimental Approach
Report
Share
Report
Share
Download to read offline
Recommended
Study of Quality of Raw Materials and General methods of analysis of Raw mate...

Study of Quality of Raw Materials and General methods of analysis of Raw mate...Raghavendra institute of pharmaceutical education and research .
Recommended
Study of Quality of Raw Materials and General methods of analysis of Raw mate...

Study of Quality of Raw Materials and General methods of analysis of Raw mate...Raghavendra institute of pharmaceutical education and research .
QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...

QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...CARE COLLEGE OF PHARMACY
More Related Content
What's hot
QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...

QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...CARE COLLEGE OF PHARMACY
What's hot (20)
ICH guidelines on impurities in new drug products.pptx

ICH guidelines on impurities in new drug products.pptx
impurities of new drug products based on ICH guidelines(Q3BR2)

impurities of new drug products based on ICH guidelines(Q3BR2)
QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...

QUANTITATIVE DETERMINATION OF PRESERVATIVES, EMULSIFIERS, AND COLOURING MATER...
Similar to Forced Degradation Study
Similar to Forced Degradation Study (20)
Extractables and leachables regulatory perspectives

Extractables and leachables regulatory perspectives
DRUG DISSOLUTION, BIO-AVAILABILITY AND IVIVC DEVELOPMENT

DRUG DISSOLUTION, BIO-AVAILABILITY AND IVIVC DEVELOPMENT
Analytical Testing Specifications-Presentation No. 2

Analytical Testing Specifications-Presentation No. 2
Analysis of parenteral dosage forms bjl final seminar

Analysis of parenteral dosage forms bjl final seminar
Recently uploaded
Gallbladder Double-Diverticular: A Case Report المرارة مزدوجة التج: تقرير حالة

Gallbladder Double-Diverticular: A Case Report المرارة مزدوجة التج: تقرير حالةMohamad محمد Al-Gailani الكيلاني
Unlocking Holistic Wellness: Addressing Depression, Mental Well-Being, and St...

Unlocking Holistic Wellness: Addressing Depression, Mental Well-Being, and St...Health Kinesiology Natural Bioenergetics
Recently uploaded (20)
Capillary Blood Collection Tubes: The Complete Guidebook

Capillary Blood Collection Tubes: The Complete Guidebook
The Clean Living Project Episode 24 - Subconscious

The Clean Living Project Episode 24 - Subconscious
Gallbladder Double-Diverticular: A Case Report المرارة مزدوجة التج: تقرير حالة

Gallbladder Double-Diverticular: A Case Report المرارة مزدوجة التج: تقرير حالة
NDCT Rules, 2019: An Overview | New Drugs and Clinical Trial Rules 2019

NDCT Rules, 2019: An Overview | New Drugs and Clinical Trial Rules 2019
Signs It’s Time for Physiotherapy Sessions Prioritizing Wellness

Signs It’s Time for Physiotherapy Sessions Prioritizing Wellness
Tips and tricks to pass the cardiovascular station for PACES exam

Tips and tricks to pass the cardiovascular station for PACES exam
The Orbit & its contents by Dr. Rabia I. Gandapore.pptx

The Orbit & its contents by Dr. Rabia I. Gandapore.pptx
SEMESTER-V CHILD HEALTH NURSING-UNIT-1-INTRODUCTION.pdf

SEMESTER-V CHILD HEALTH NURSING-UNIT-1-INTRODUCTION.pdf
Get the best psychology treatment in Indore at Gokuldas Hospital

Get the best psychology treatment in Indore at Gokuldas Hospital
Hemodialysis: Chapter 1, Physiological Principles of Hemodialysis - Dr.Gawad

Hemodialysis: Chapter 1, Physiological Principles of Hemodialysis - Dr.Gawad
Unveiling Alcohol Withdrawal Syndrome: exploring it's hidden depths

Unveiling Alcohol Withdrawal Syndrome: exploring it's hidden depths
Report Back from SGO: What’s the Latest in Ovarian Cancer?

Report Back from SGO: What’s the Latest in Ovarian Cancer?
Histology of Epithelium - Dr Muhammad Ali Rabbani - Medicose Academics

Histology of Epithelium - Dr Muhammad Ali Rabbani - Medicose Academics
Failure to thrive in neonates and infants + pediatric case.pptx

Failure to thrive in neonates and infants + pediatric case.pptx
TEST BANK for The Nursing Assistant Acute, Subacute, and Long-Term Care, 6th ...

TEST BANK for The Nursing Assistant Acute, Subacute, and Long-Term Care, 6th ...
Unlocking Holistic Wellness: Addressing Depression, Mental Well-Being, and St...

Unlocking Holistic Wellness: Addressing Depression, Mental Well-Being, and St...
Forced Degradation Study
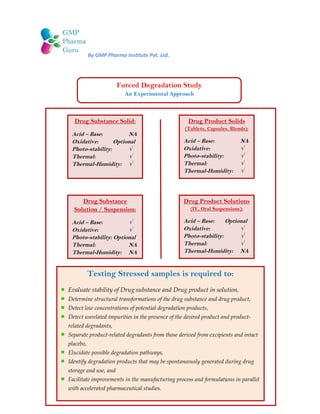
- 1. By GMP Pharma Institute Pvt. Ltd. Drug Substance Solid: Acid – Base: NA Oxidative: Optional Photo-stability: √ Thermal: √ Thermal-Humidity: √ Drug Substance Solution / Suspension: Acid – Base: √ Oxidative: √ Photo-stability: Optional Thermal: NA Thermal-Humidity: NA Drug Product Solids (Tablets, Capsules, Blends): Acid – Base: NA Oxidative: √ Photo-stability: √ Thermal: √ Thermal-Humidity: √ Drug Product Solutions (IV, Oral Suspensions): Acid – Base: Optional Oxidative: √ Photo-stability: √ Thermal: √ Thermal-Humidity: NA Forced Degradation Study An Experimental Approach Testing Stressed samples is required to: Evaluate stability of Drug substance and Drug product in solution, Determine structural transformations of the drug substance and drug product, Detect low concentrations of potential degradation products, Detect unrelated impurities in the presence of the desired product and product- related degradants, Separate product-related degradants from those derived from excipients and intact placebo, Elucidate possible degradation pathways, Identify degradation products that may be spontaneously generated during drug storage and use, and Facilitate improvements in the manufacturing process and formulations in parallel with accelerated pharmaceutical studies.