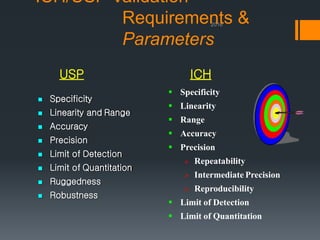
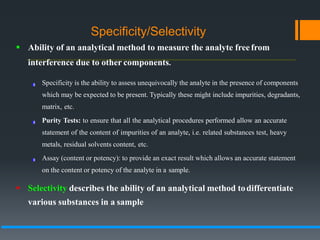
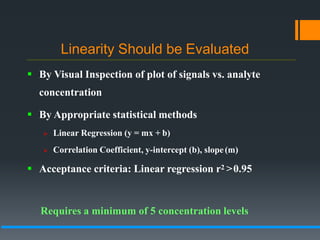
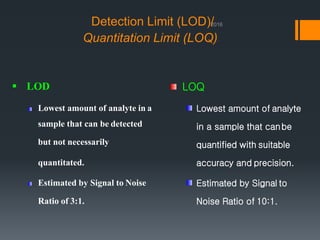
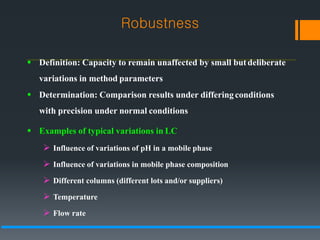
This document discusses guidelines for validating analytical methods from the International Council for Harmonisation (ICH). It defines method validation as demonstrating that analytical procedures are suitable for their intended use. Key parameters of method validation discussed include specificity, linearity, range, accuracy, precision, detection and quantitation limits, ruggedness and robustness. The guidelines provide criteria for acceptance in each parameter area to ensure analytical methods are suitable to support the quality and potency of pharmaceutical products.