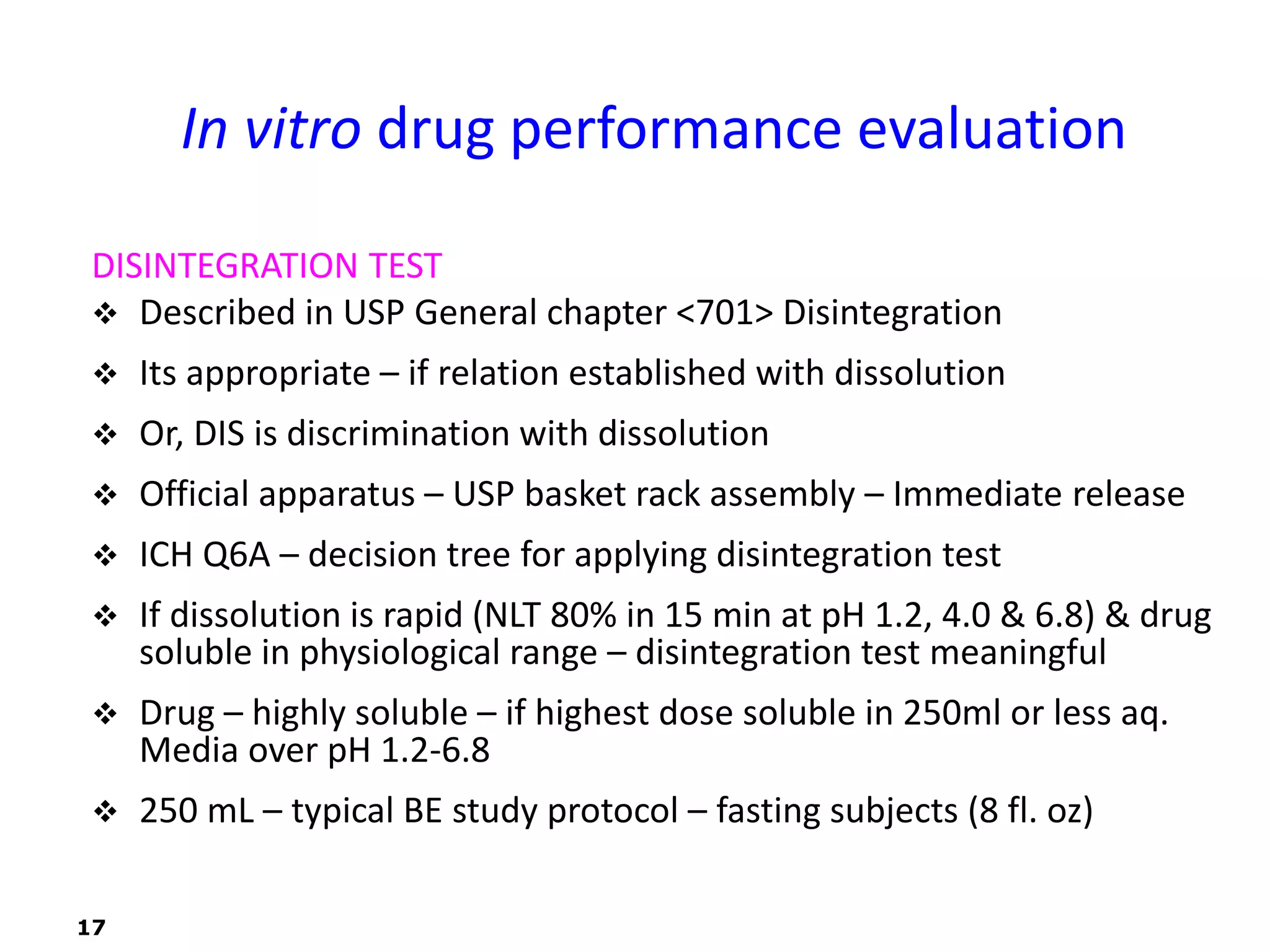
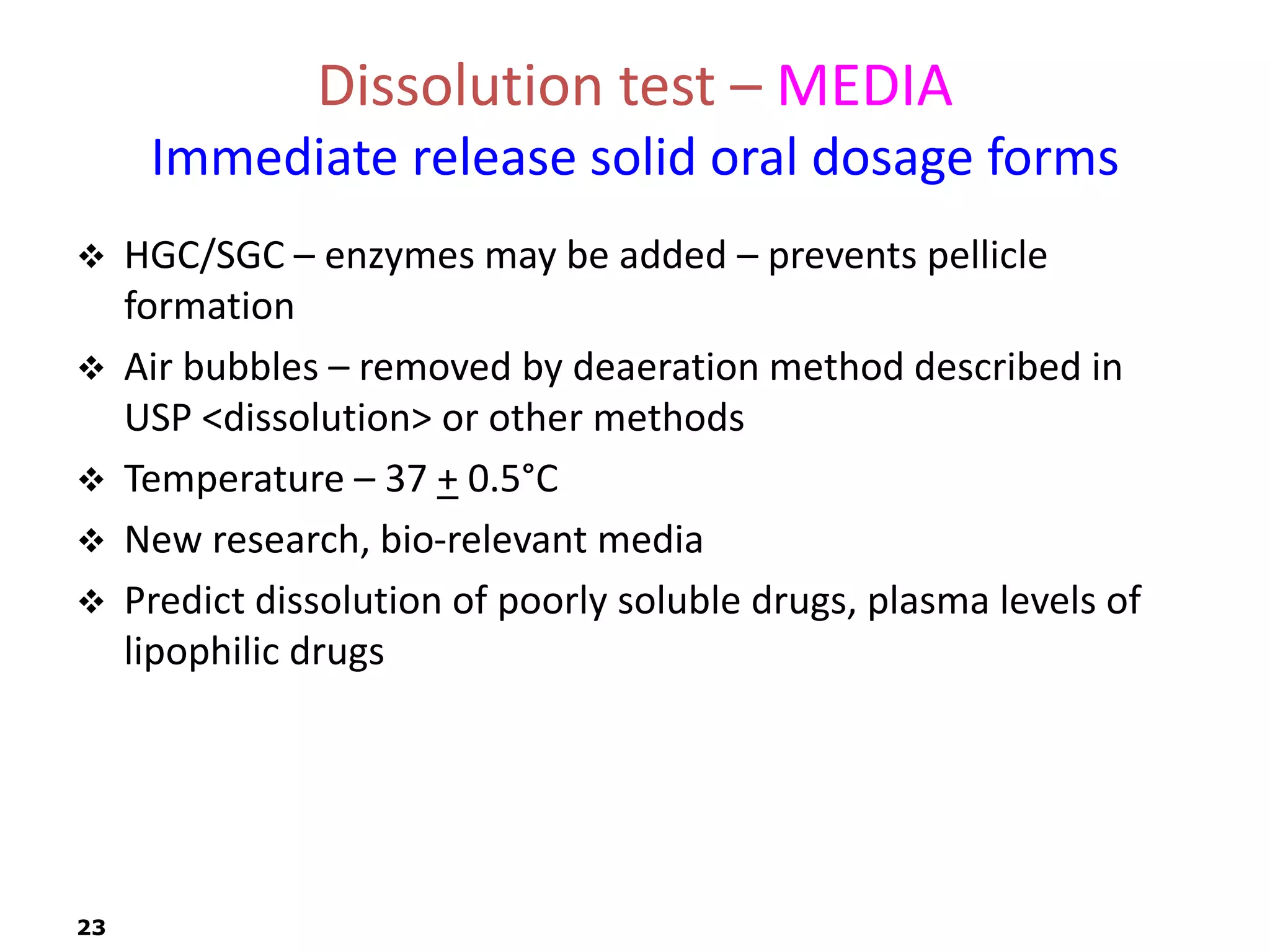
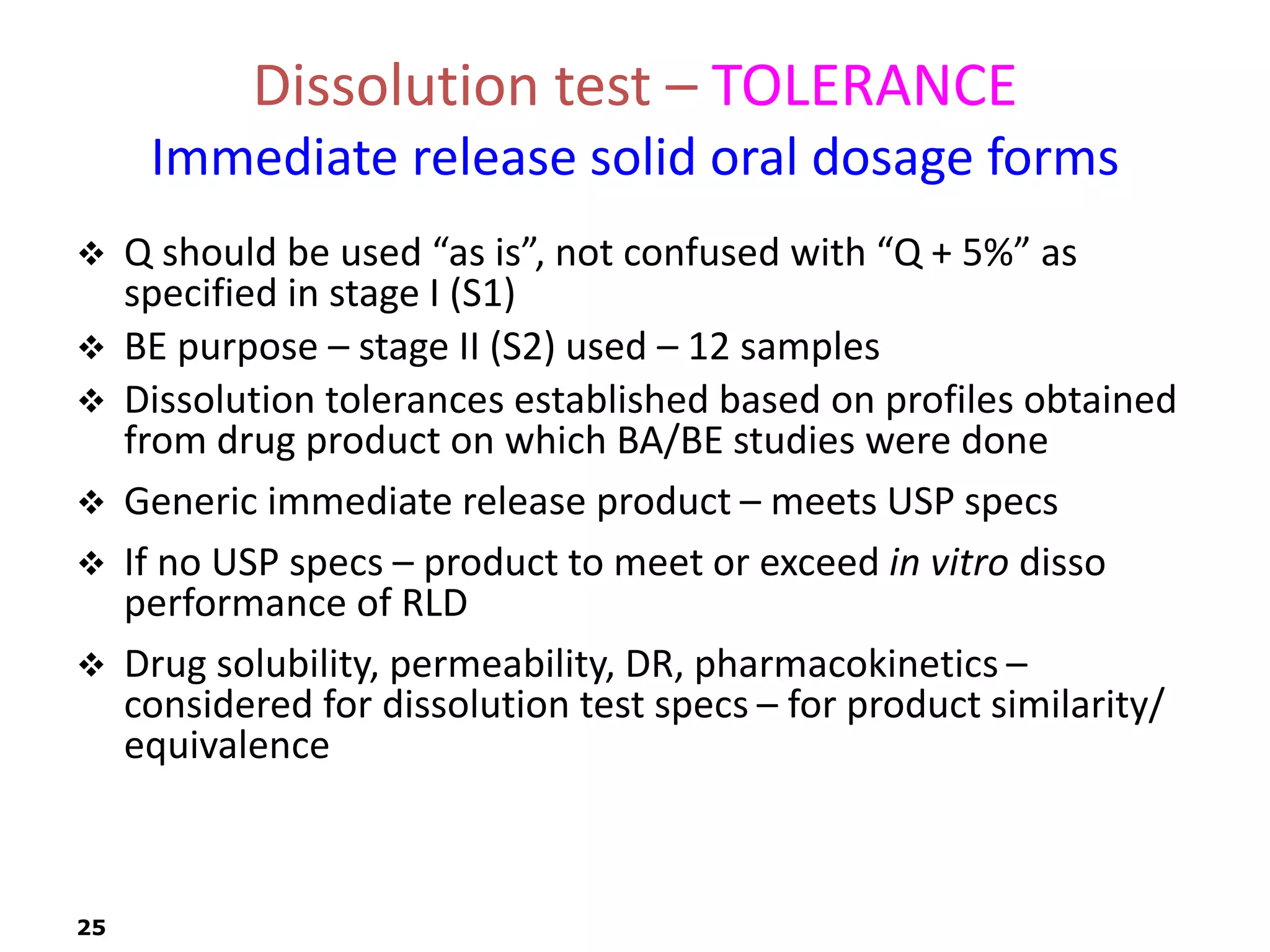
This document discusses in vitro drug product performance characterization and dissolution testing of solid oral dosage forms. It describes the importance of in vitro testing in predicting in vivo drug absorption and performance. The key factors that can affect drug dissolution are described, including drug substance properties, formulation composition, manufacturing processes, and test conditions. Common dissolution apparatus, media, and acceptance tolerances used in testing immediate release solid oral drug products are also summarized.






![FACTORS RELATED TO DRUG SUBSTANCE
Dissolution - Solubilization of drug into dissolution medium
Control – affinity b/n solid & disso lutionmedium, as diffusion of drug into
bulk liquid media
Noyes and Whitney equation
dm/dt = K (Cs-Ct)
(Cs-Ct) – conc gradient b/n diffusion layer & bulk soln
Brunner & Tolloczko incorporated S
dm/dt = K’ x S (Cs-Ct)
S – Surface area, K’ – constant to chemical subs
Brunner expanded scope – included Nernst’s theory
Dc/dt = [DS/Vh] x (Cs-Ct)
D – Diffusion coefficient, h – thickness of diffusion layer, V –dissolution
volume
Drug dissolution – inflUenced by solubility, diffusivity, SA and solution
hydrodynamics
8](https://image.slidesharecdn.com/invitrodrugproductperformance-230714041538-7df2358a/75/In-Vitro-Drug-Product-Performance-ppt-8-2048.jpg)