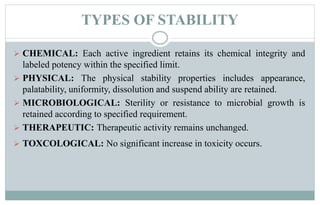
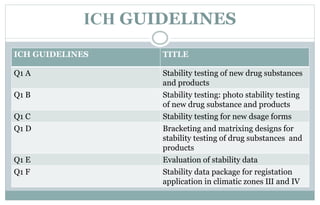
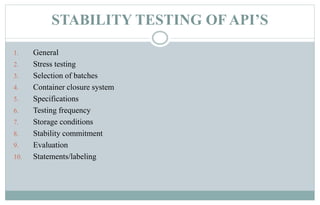
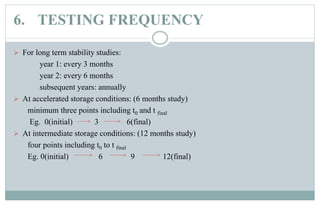
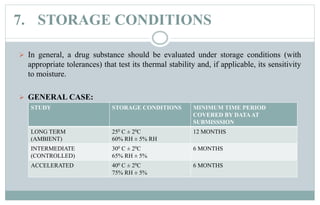
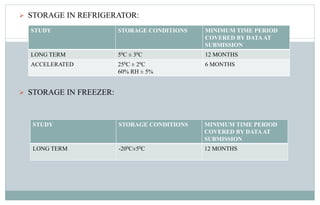
Stability testing is used to provide evidence of how the quality of a drug substance or product varies over time under environmental conditions like temperature, humidity, and light. Guidelines provide recommendations on conducting stability tests including storing samples under long-term, intermediate, and accelerated conditions and specifying the testing frequency. Stability tests evaluate attributes of the drug substance or product that may change during storage. The results are used to establish a retest period to ensure the stated quality of the substance or product through the expiration date.