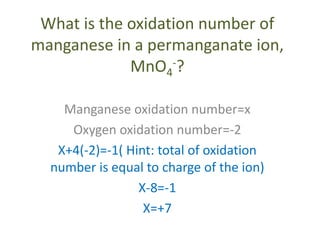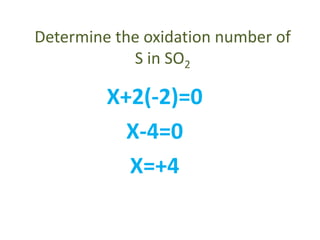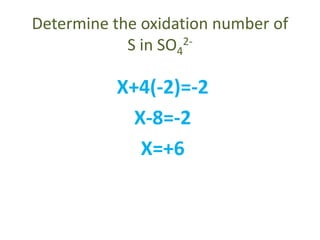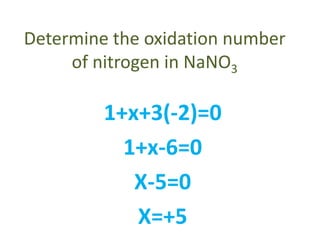This document discusses oxidation and reduction reactions and oxidation numbers. It provides rules for assigning oxidation numbers to elements in compounds and examples of using the rules to determine oxidation numbers. The key points are:
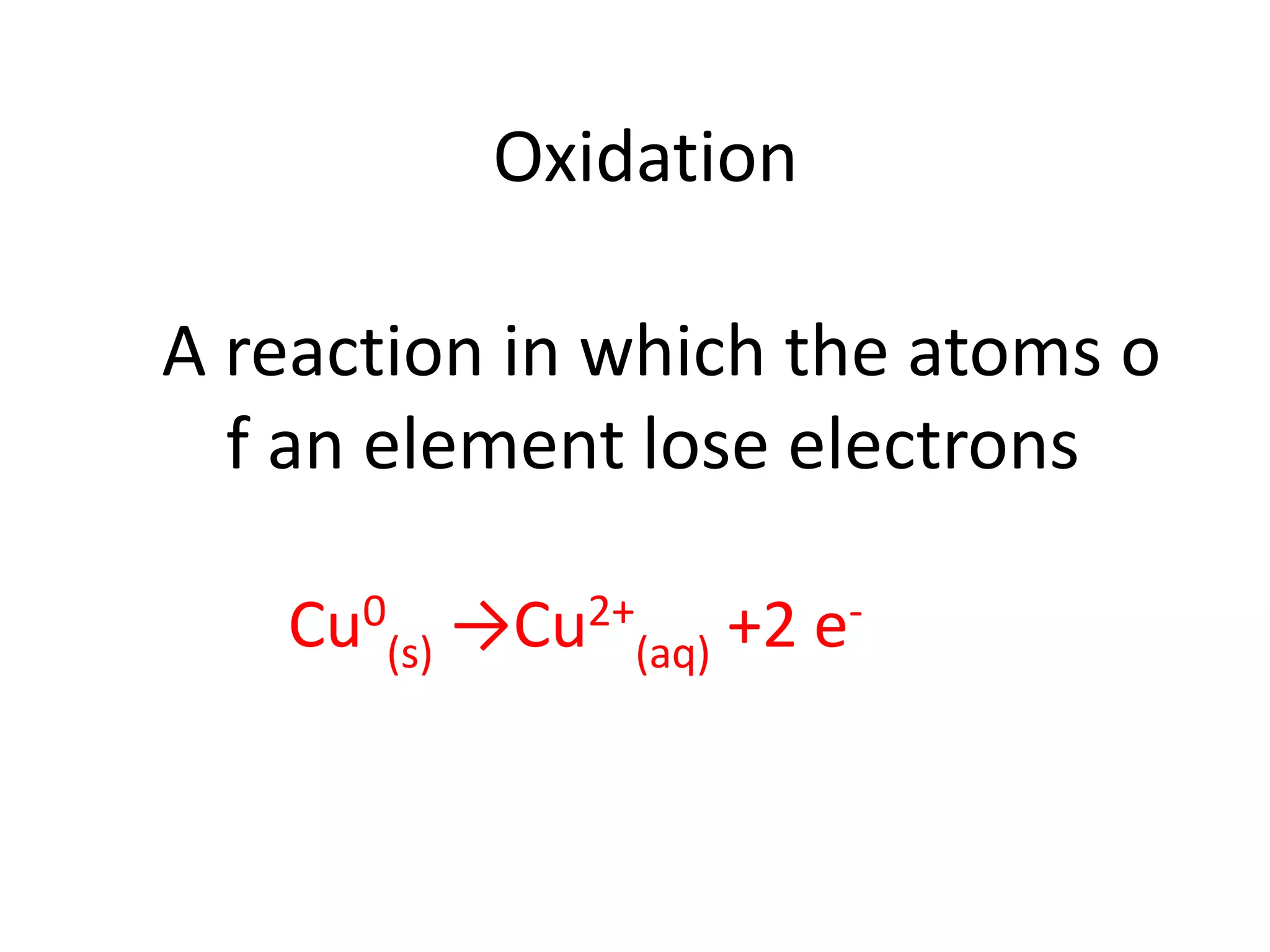
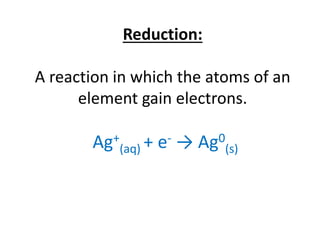
- Oxidation involves losing electrons and reduction involves gaining electrons.
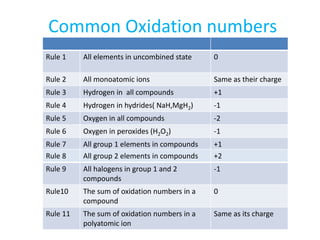
- Oxidation numbers are assigned based on arbitrary rules and are not actual charges.
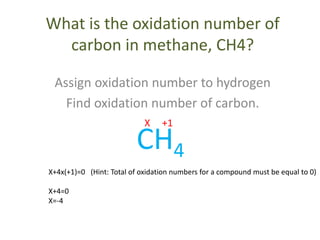
- Common rules include main group elements having an oxidation state of 0 in their elemental form, monoatomic ions having the same charge as their oxidation number, and the sum of oxidation numbers in a compound or polyatomic ion equaling the overall charge.