This document discusses exothermic and endothermic reactions, enthalpy diagrams, molar enthalpy, and example thermochemical equations and calculations:
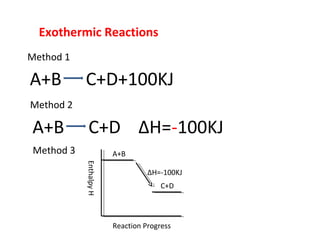
- It defines exothermic reactions as those that release heat and endothermic reactions as those that absorb heat. Method 1, 2, and 3 are given as examples of determining enthalpy change.
- It shows how to draw enthalpy diagrams that represent the energy changes in reactions over their progress.
- Molar enthalpy is defined as the enthalpy change per one mole of a substance. An example calculation is shown.
- Several example thermochemical equations and calculations are given to determine enthalpy changes, draw