The glass transition temperature of polymers is affected by several factors:
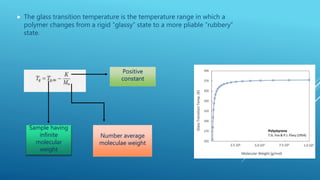
1. Molecular weight - Higher molecular weight polymers have lower mobility and higher glass transition temperatures due to increased entanglement between chains. The Fox equation models this relationship.
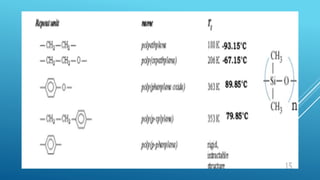
2. Chain flexibility - More flexible polymer chains with fewer steric hindrances, like polydimethylsiloxane, have lower glass transition temperatures due to increased mobility.
3. Intermolecular interactions - Stronger interactions between polymer chains increase molecular crowding and packing, raising the glass transition temperature. Changes to polymer structure can alter these interactions and the transition temperature.