1. The document discusses the physical properties of polymers, specifically focusing on their glass transition temperature (Tg) and the factors that influence Tg.
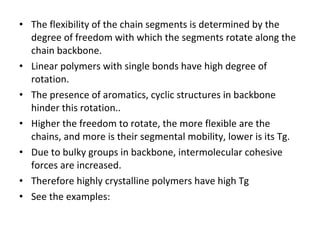
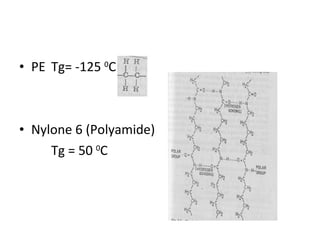
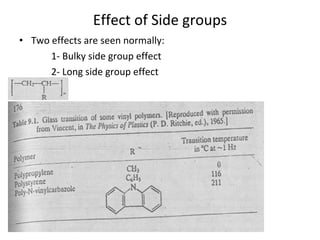
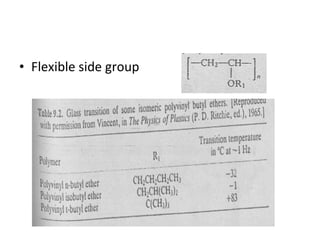
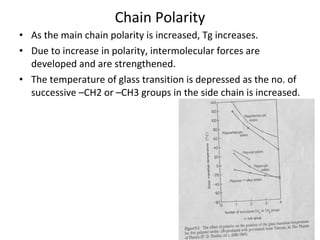
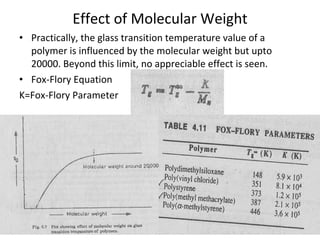
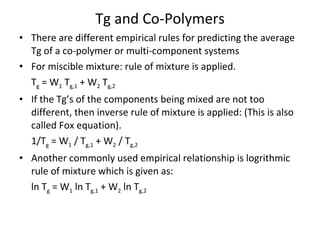
2. Tg is affected by a polymer's chemical structure, molecular weight, addition of plasticizers, and whether it is a co-polymer. Flexible chains and side groups lower Tg, while increased polarity, crystallinity and bulky groups raise Tg.
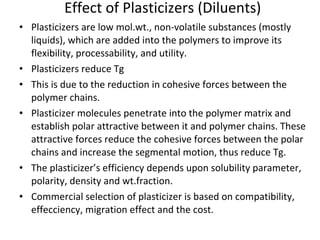
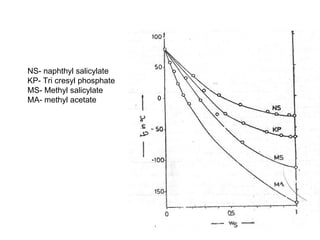
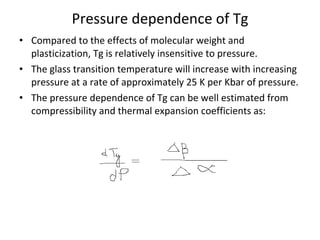
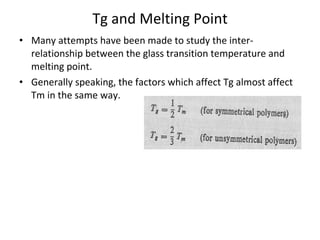
3. Plasticizers reduce intermolecular forces and increase segmental motion, thereby decreasing Tg. Tg also increases with pressure and is generally correlated with, though separate from, a polymer's melting point.