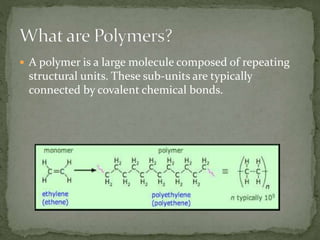
Polymer degradation is the breakdown of polymers or plastics into smaller molecules due to effects of heat, light, chemicals, or biological activity. There are several types of polymer degradation including photodegradation caused by light, thermal degradation caused by heat, chemical degradation through processes like hydrolysis and solvolysis using acids/bases, and biological degradation by microorganisms. Biodegradable polymers can break down through biological processes to give smaller molecules but require proper conditions like in compost to fully degrade.