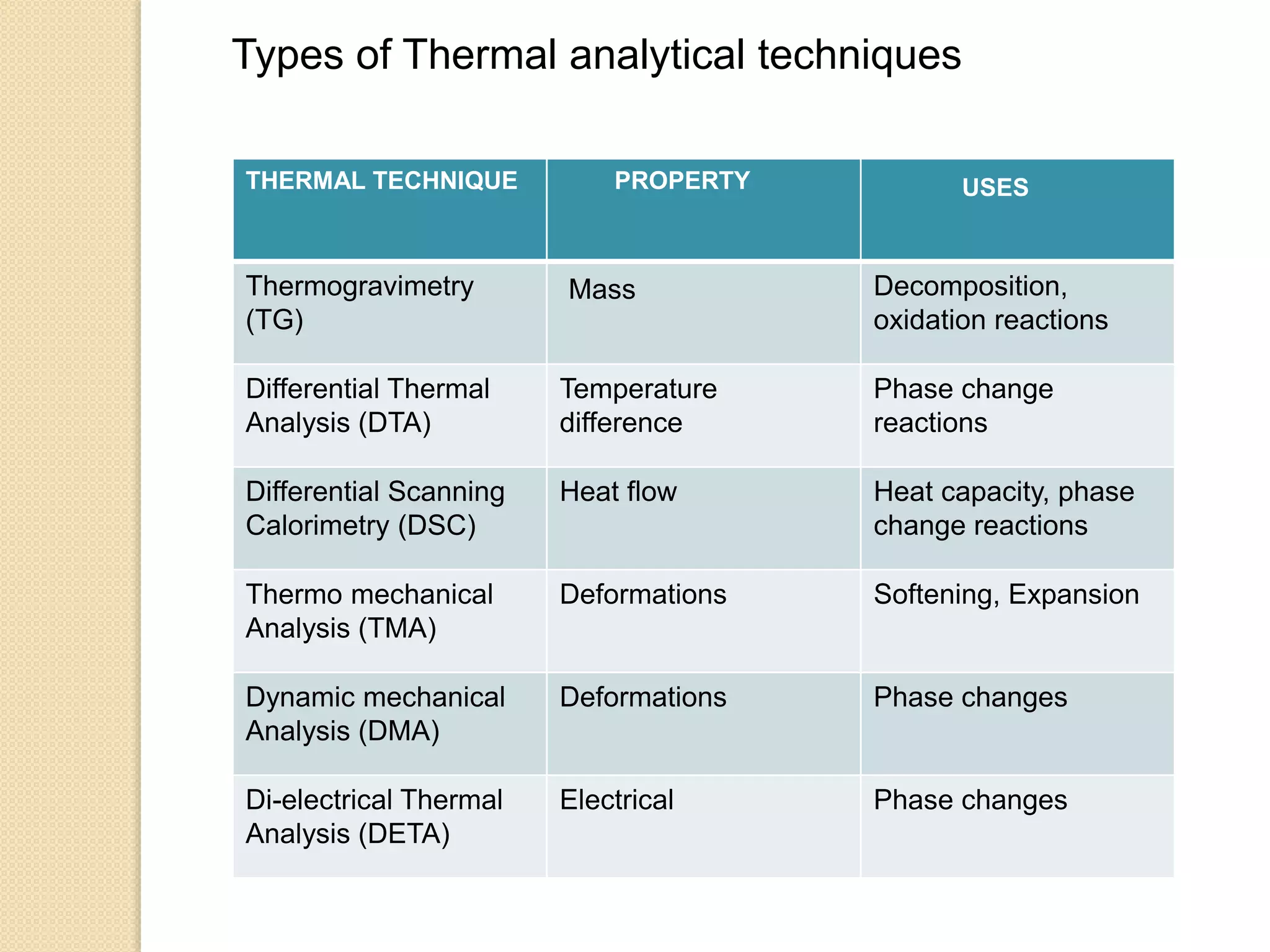
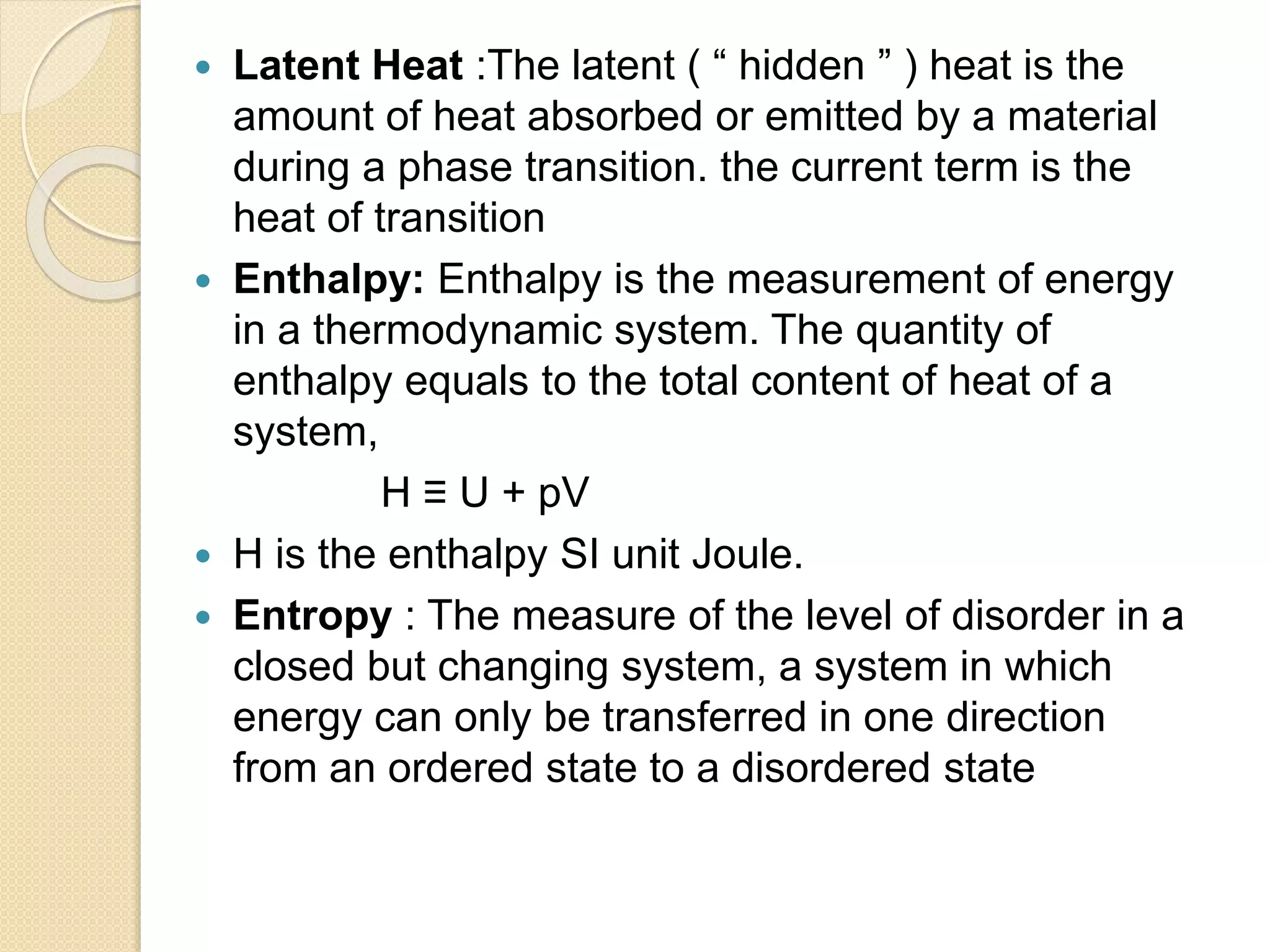
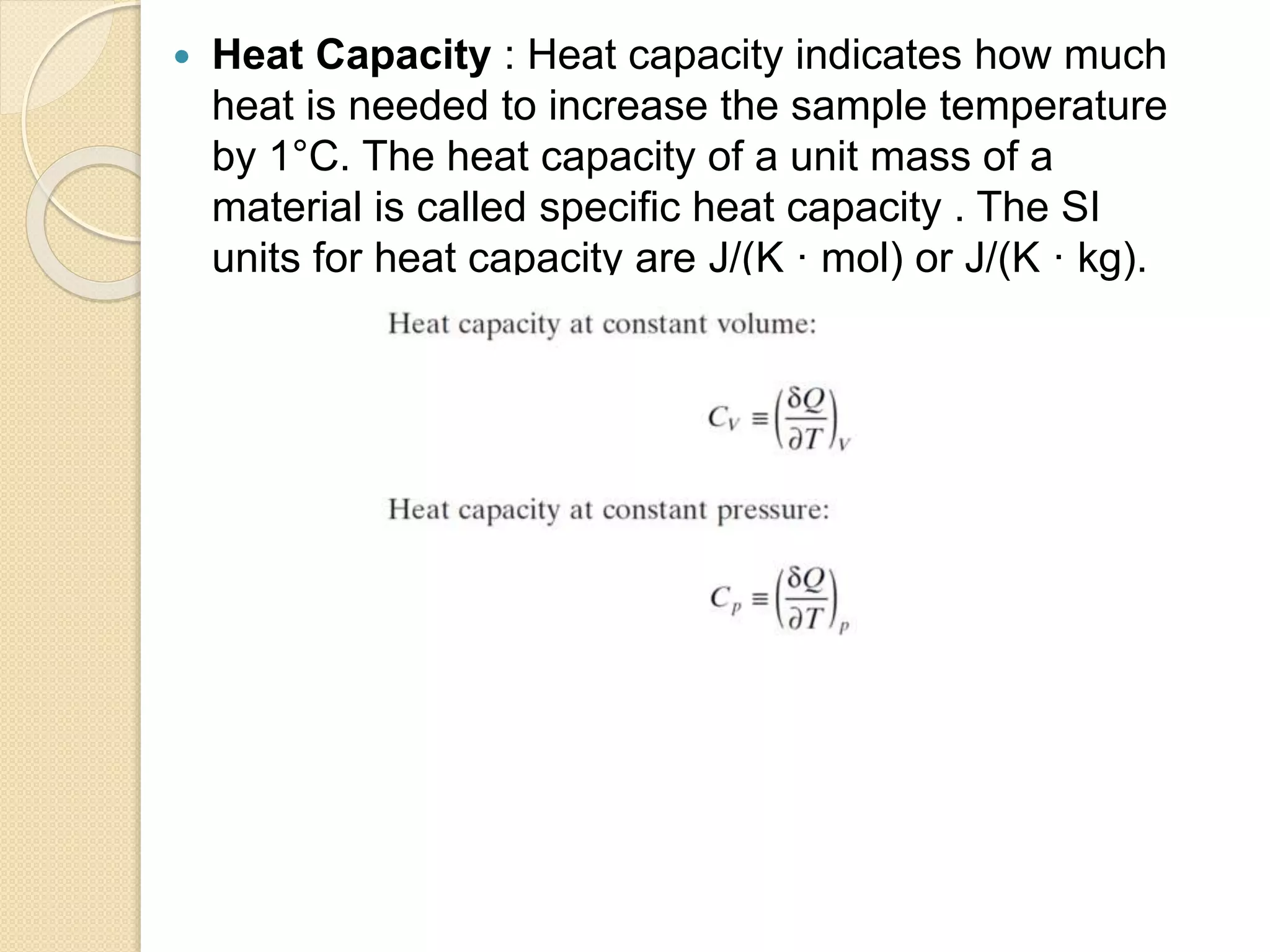
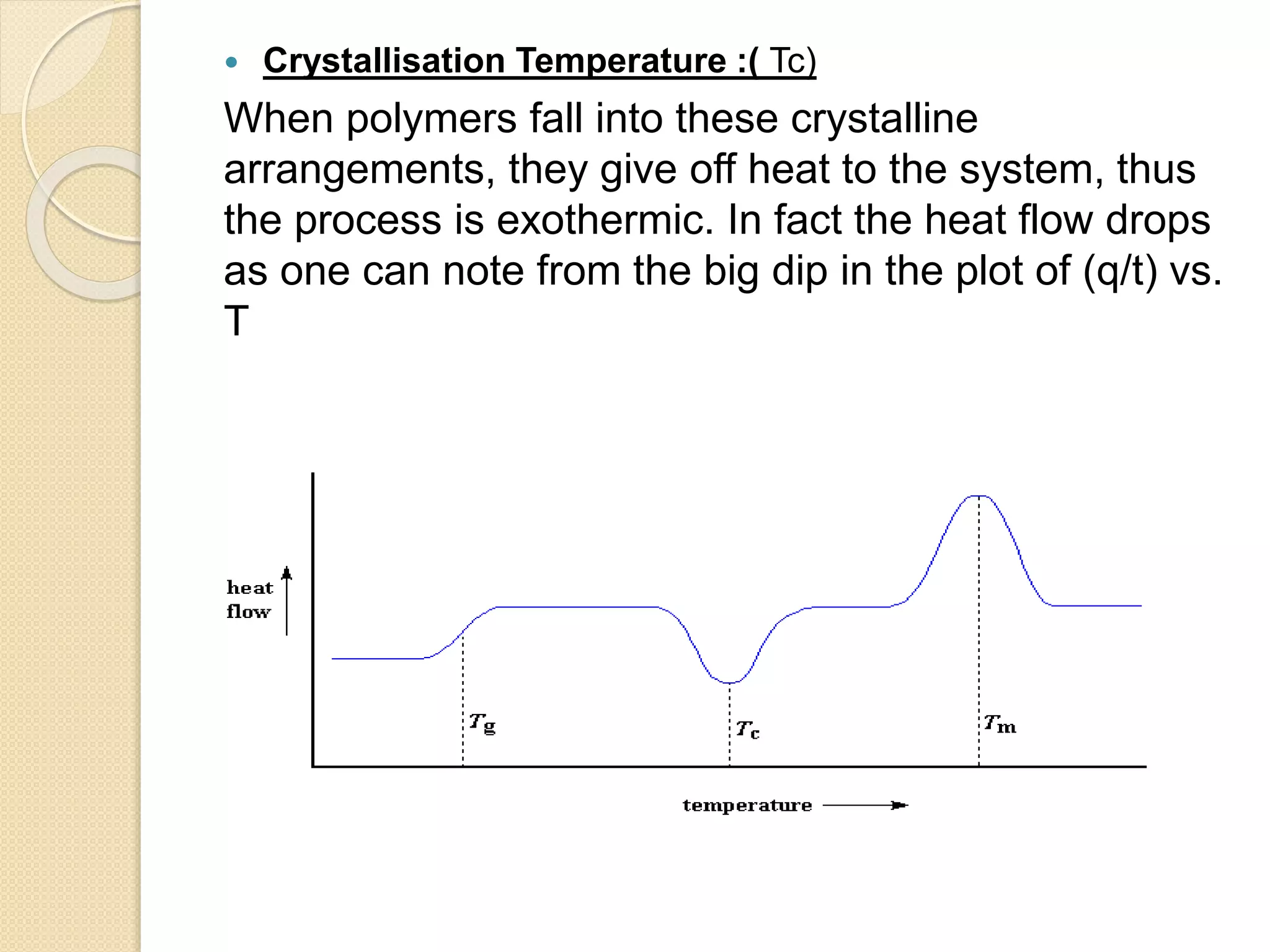
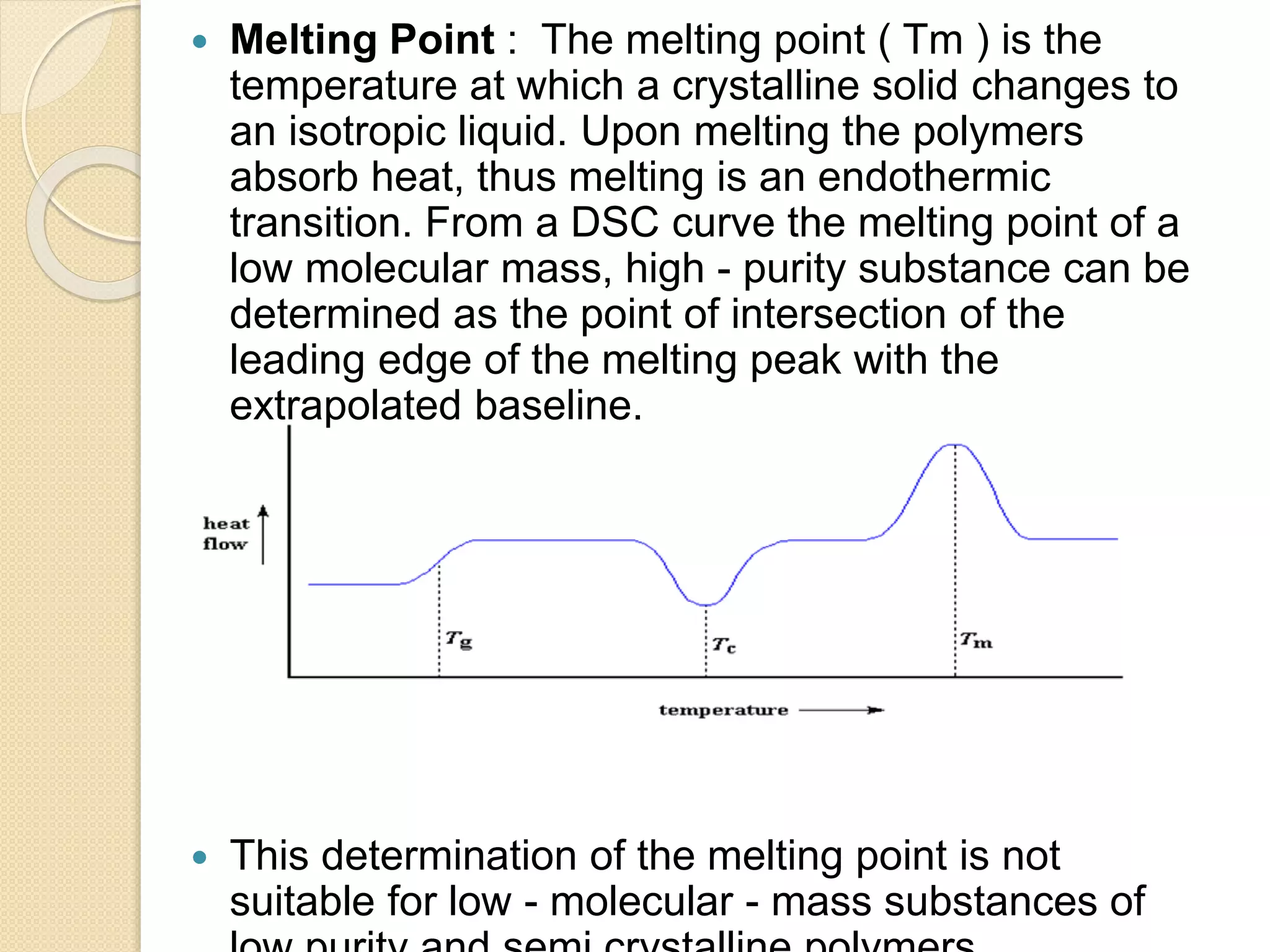
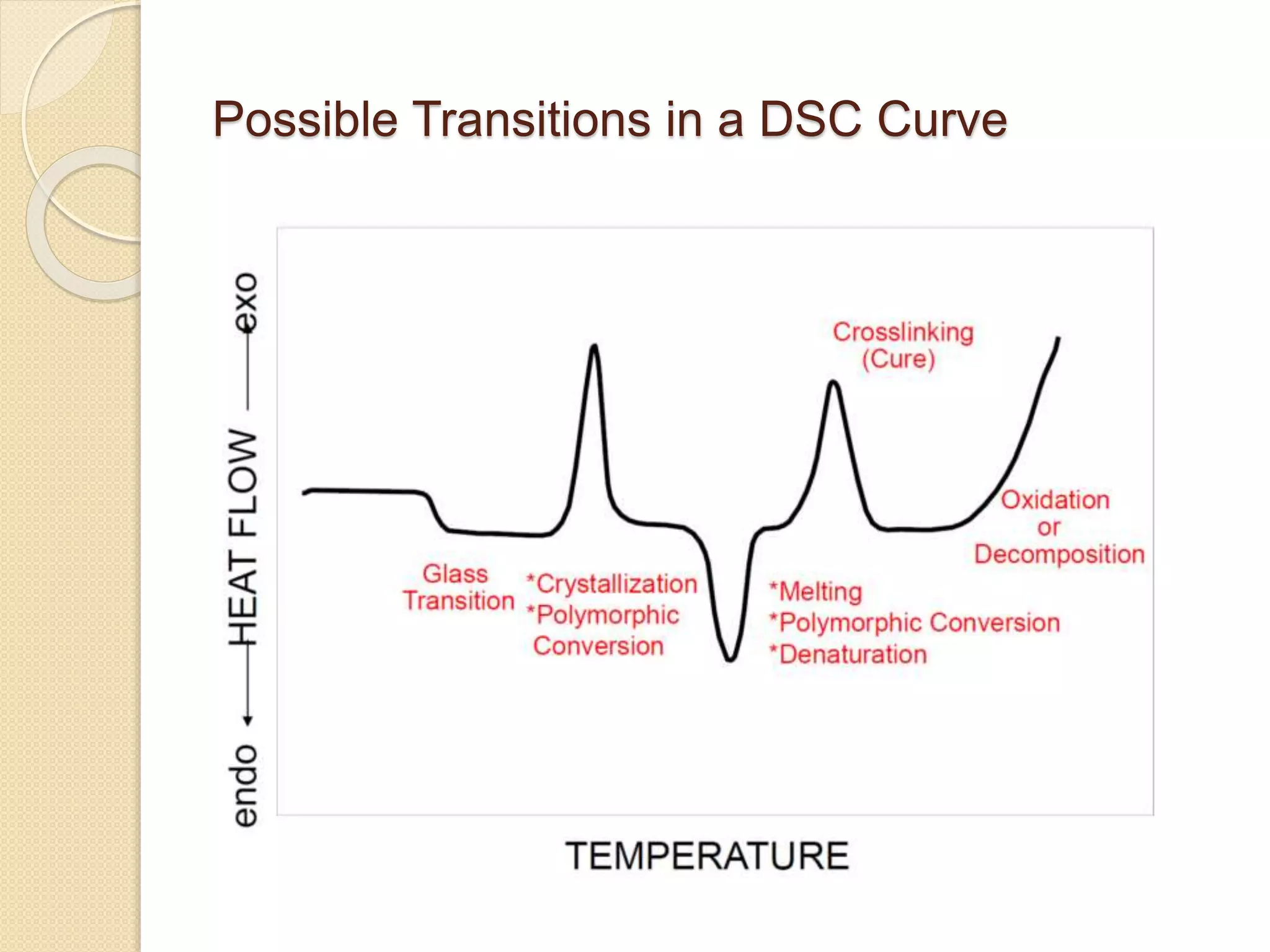
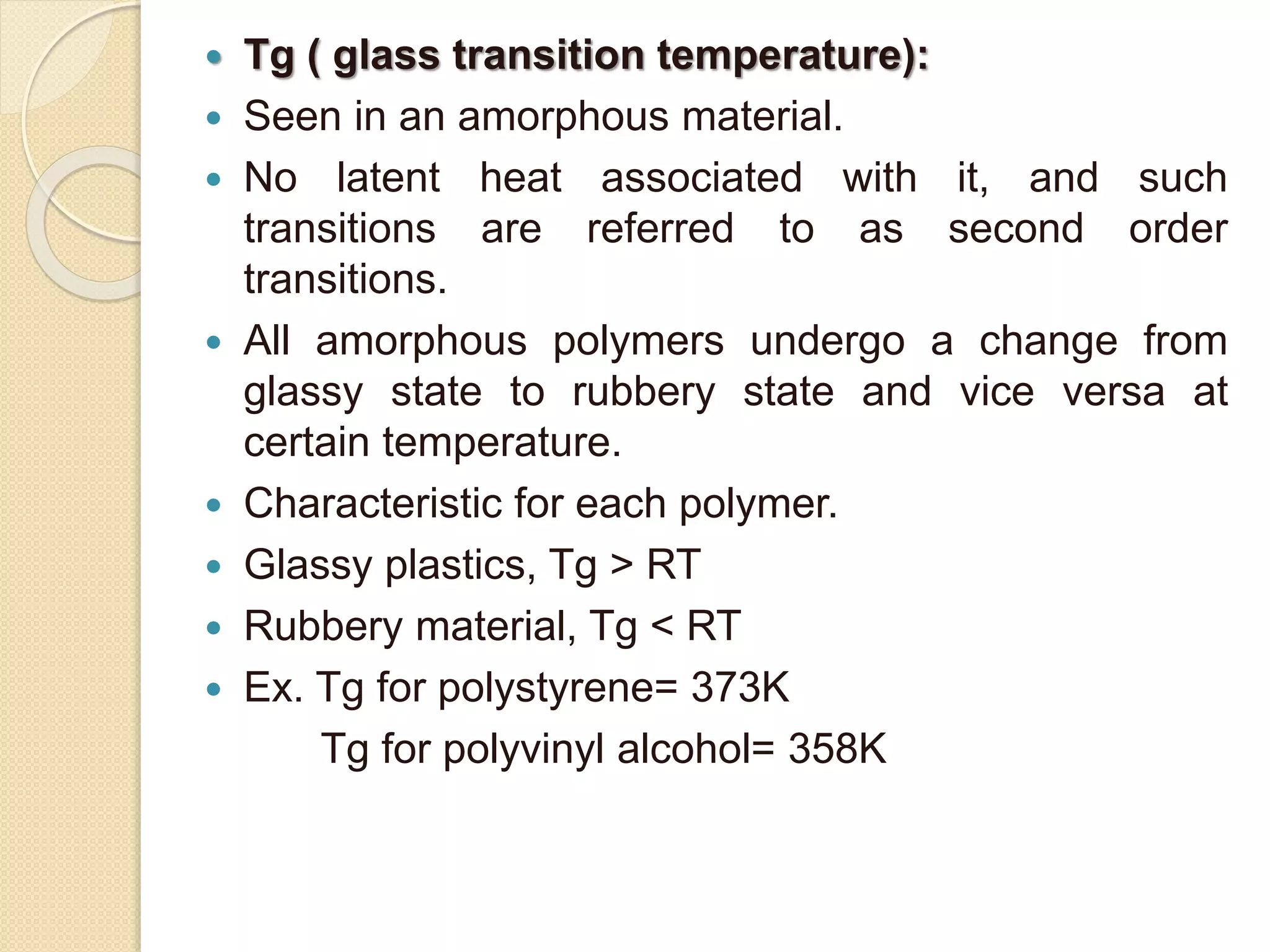
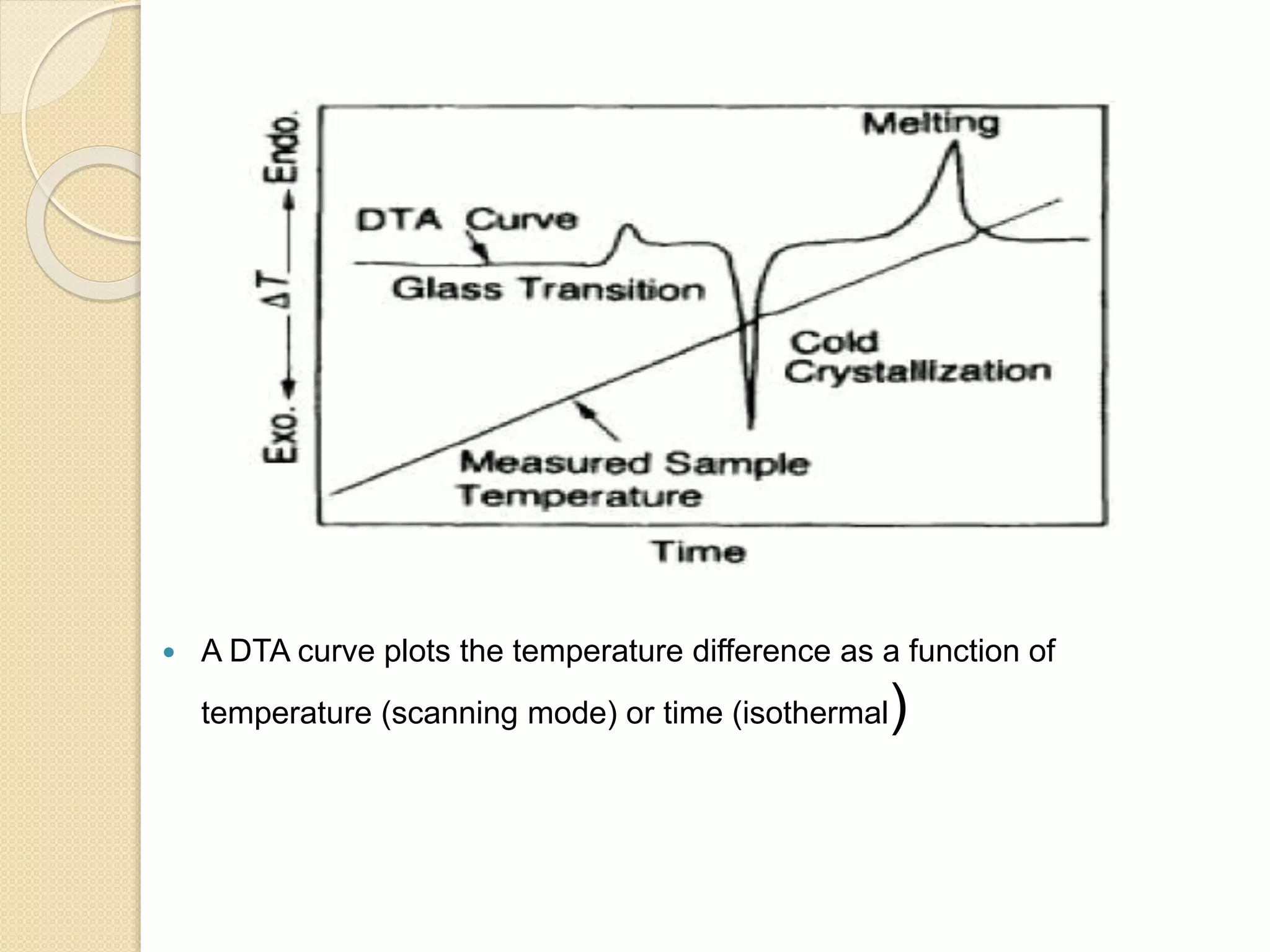
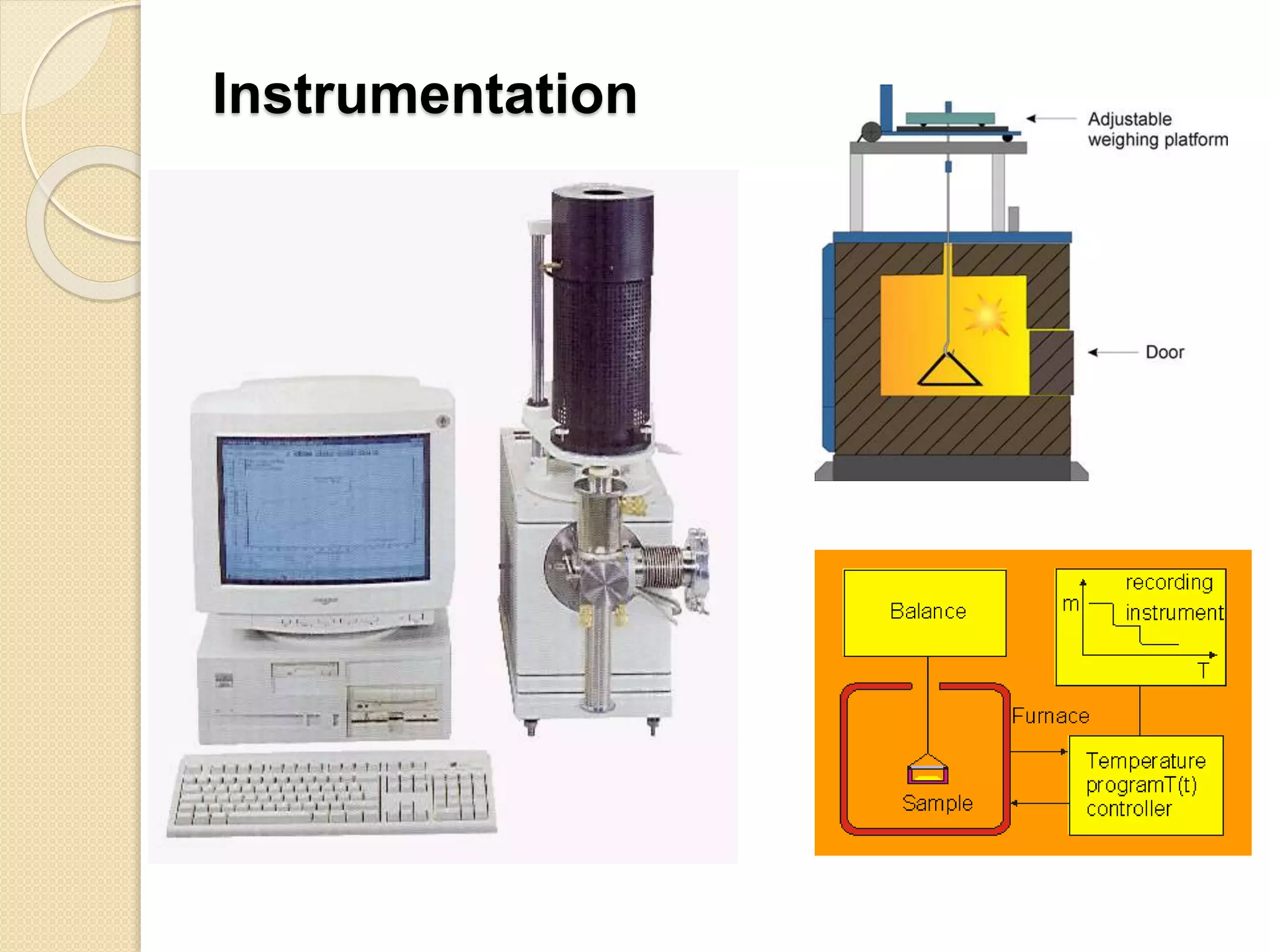
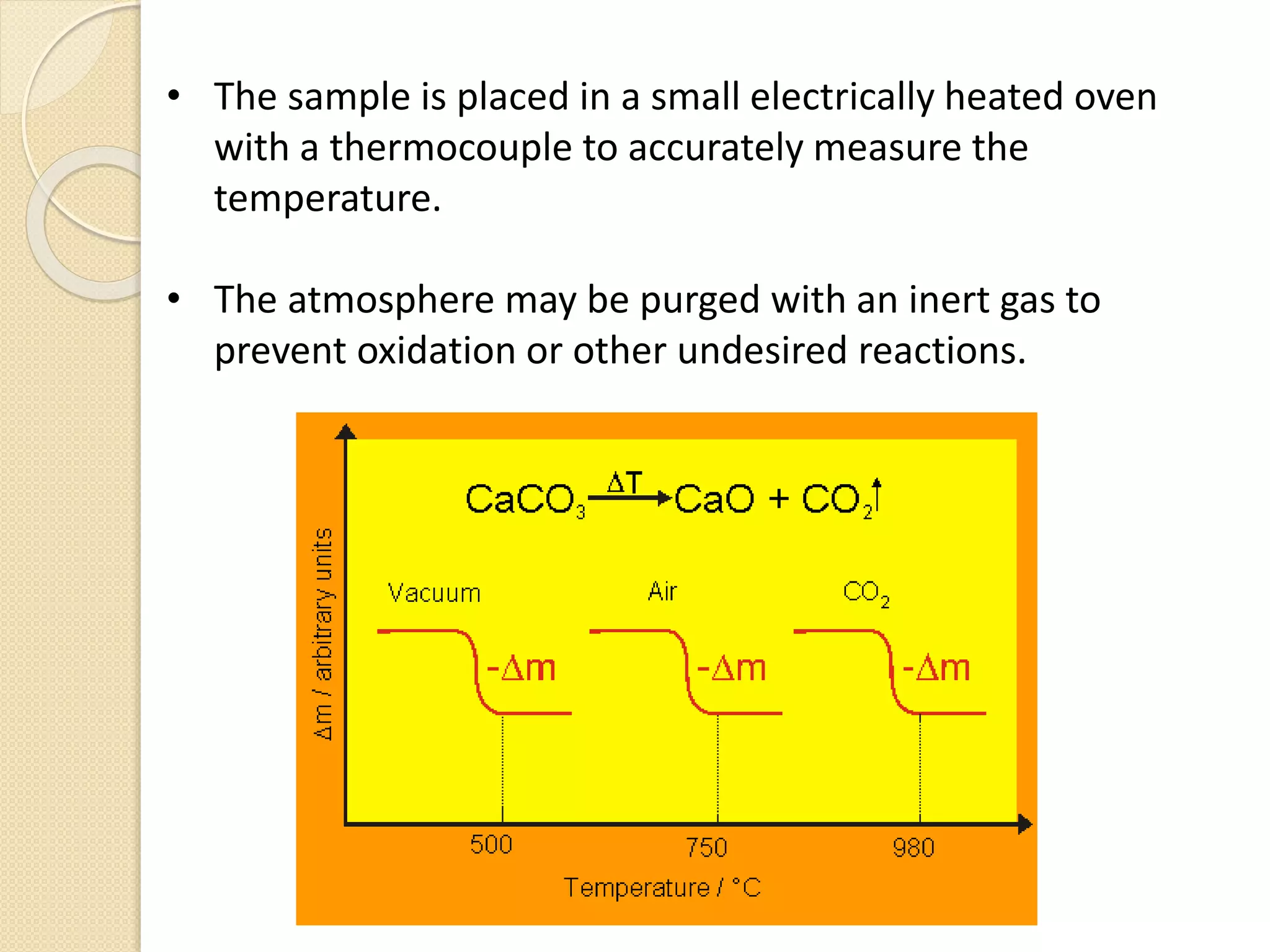
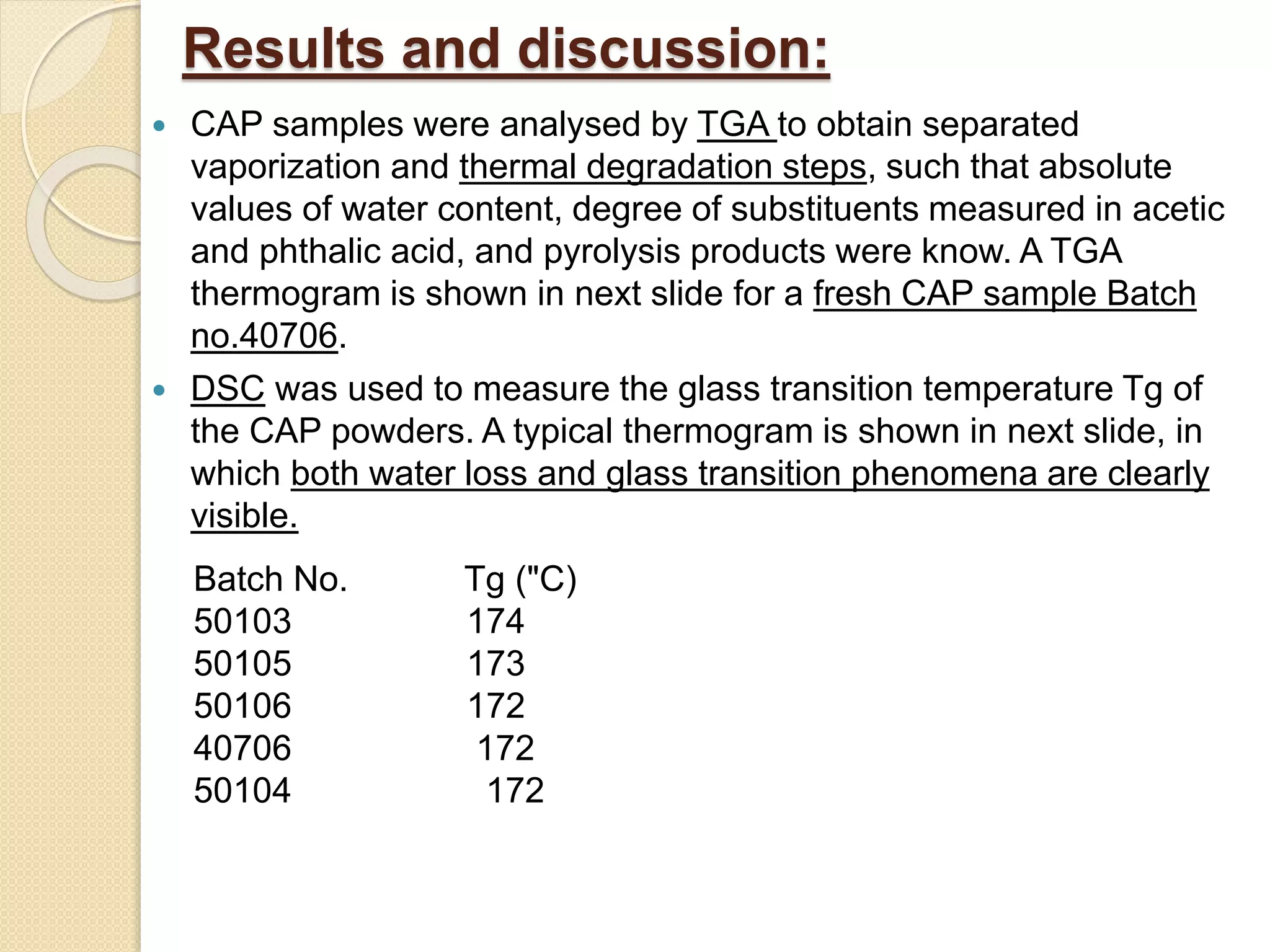
This document discusses thermal characterization techniques for polymers. It provides an overview of polymer morphology and different thermal characterization methods including DSC, DTA, TGA, and TMA. These techniques are used to measure properties like glass transition temperature, melting point, heat capacity, and thermal decomposition. The document also defines important thermal concepts and terms and provides examples of applications of these characterization methods for polymers.