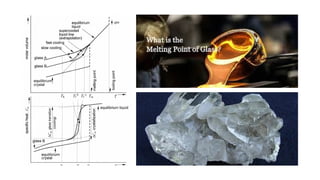
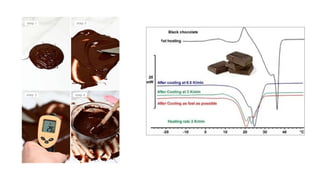
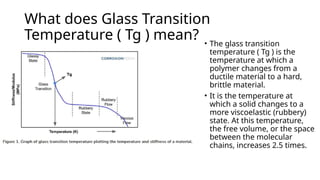
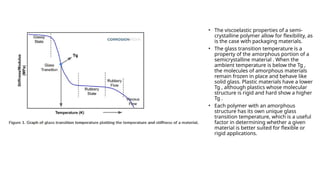
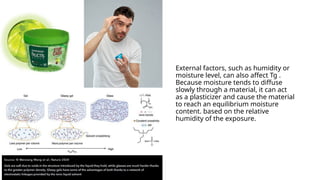
Glass transition is a phenomenon in amorphous materials like polymers and glasses, marking the transition from a viscous state to a solid. The glass transition temperature (Tg) determines the material's flexibility, with properties changing from brittle to ductile as temperature varies. Tg is crucial in materials science, impacting applications from packaging materials to automotive tires, and external factors like humidity can also influence it.