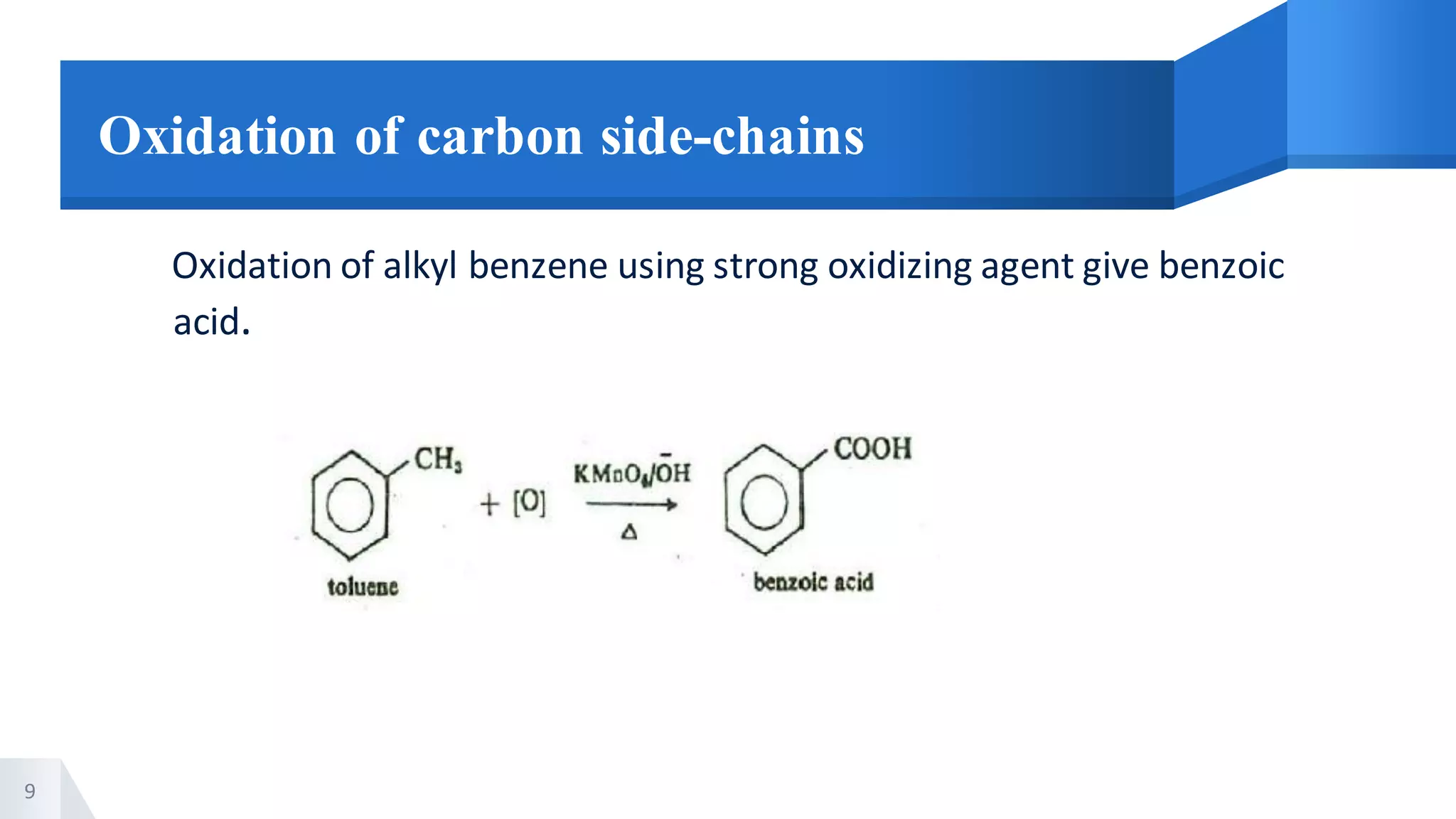
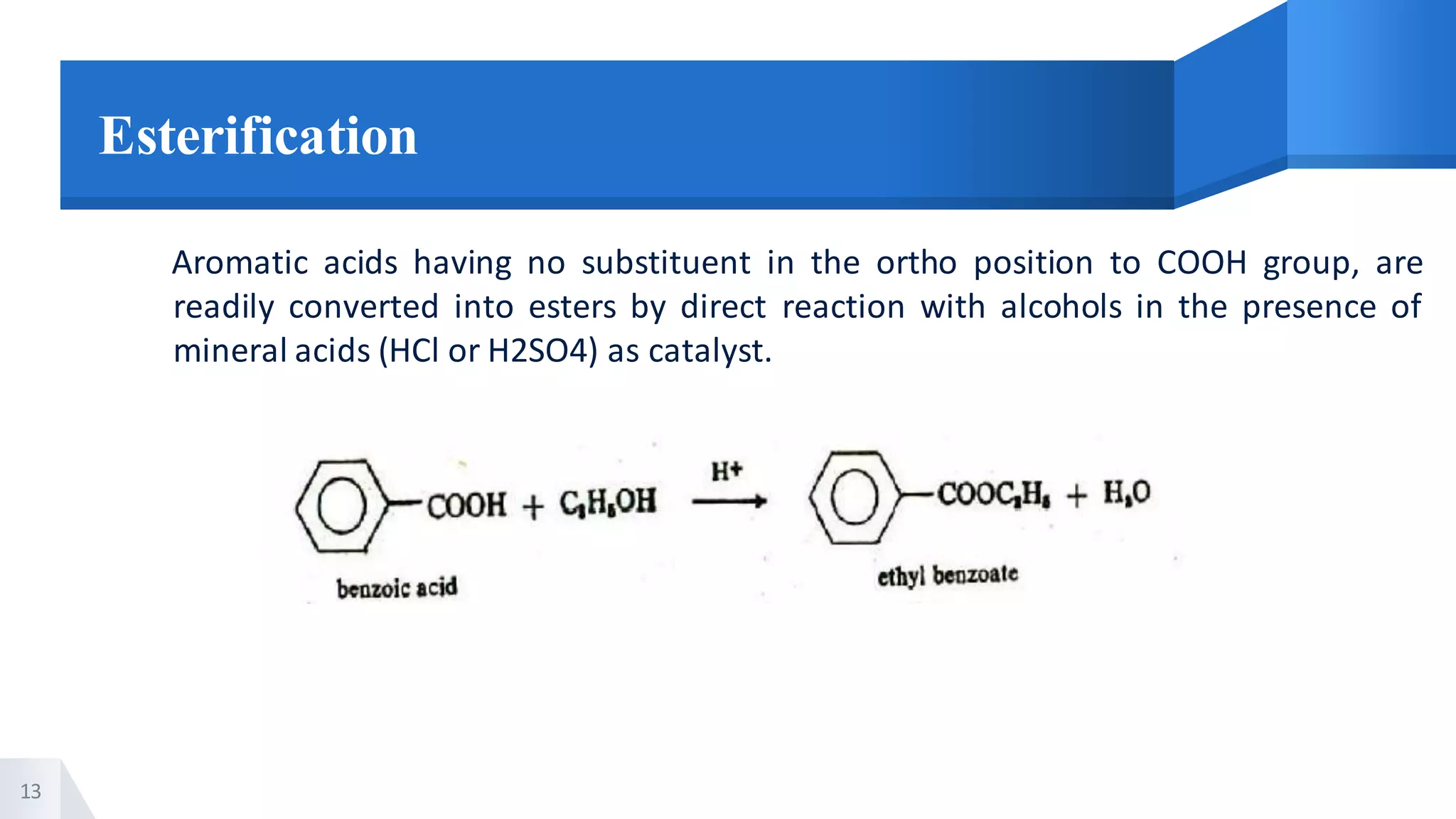
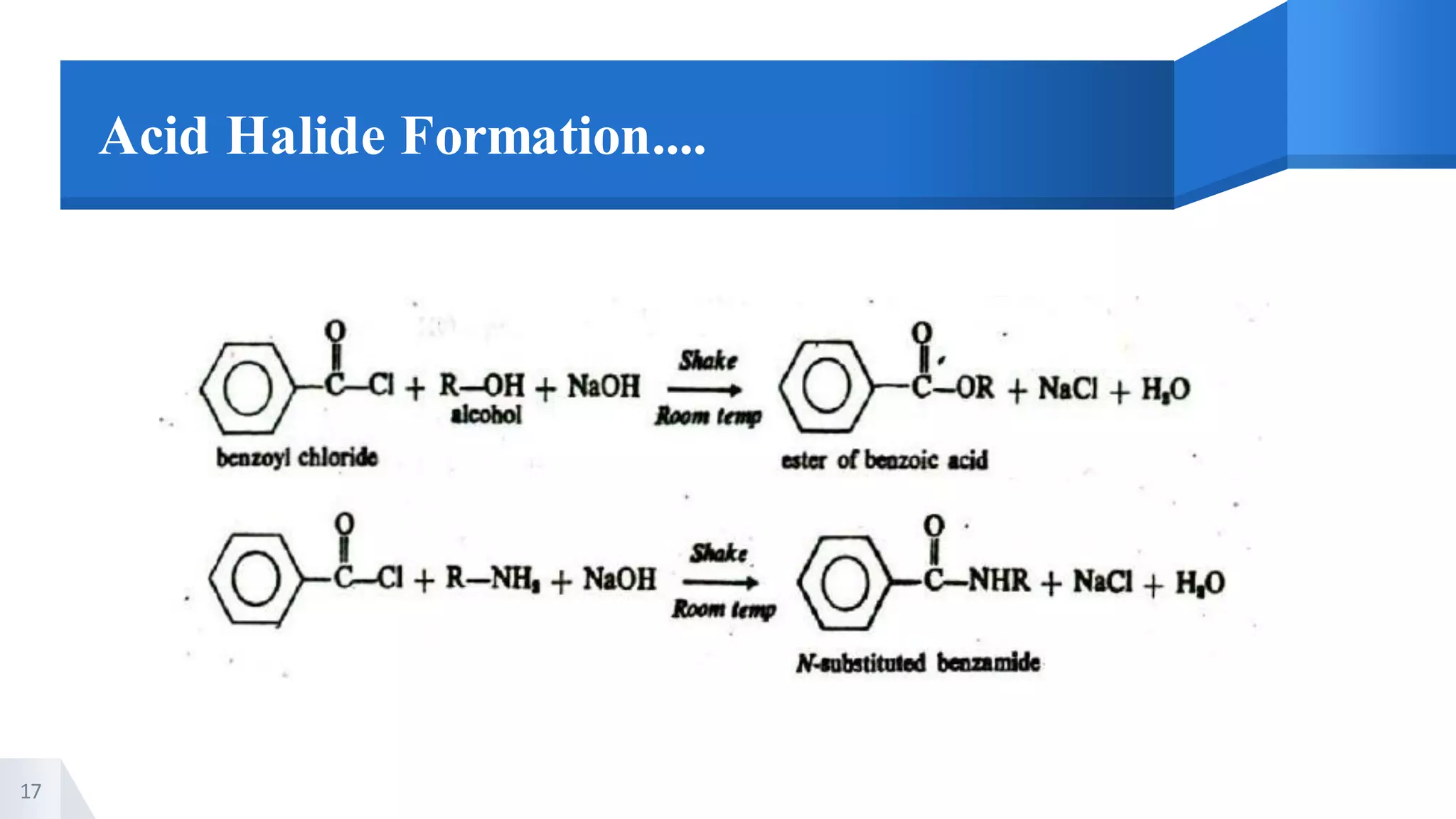
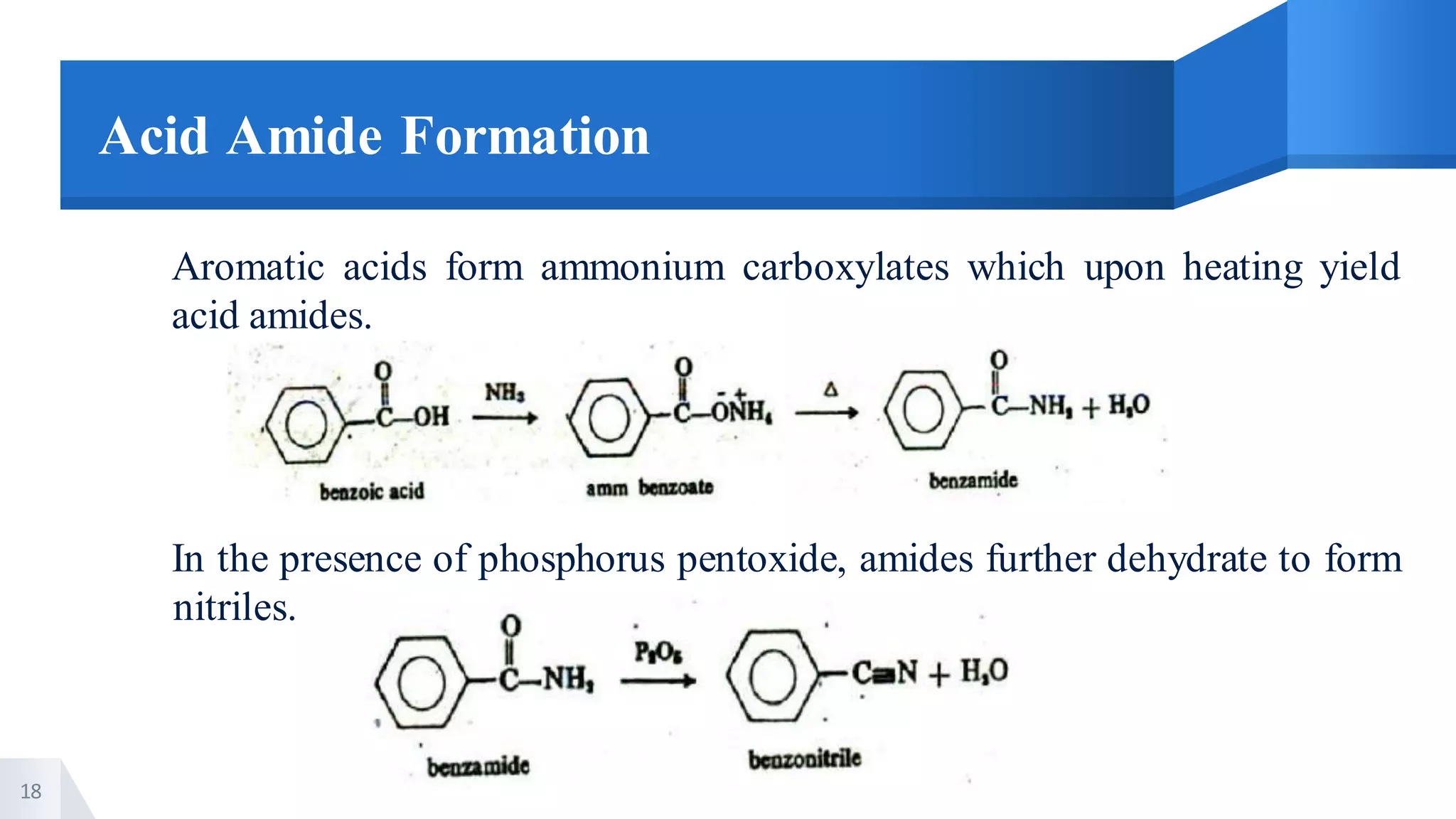
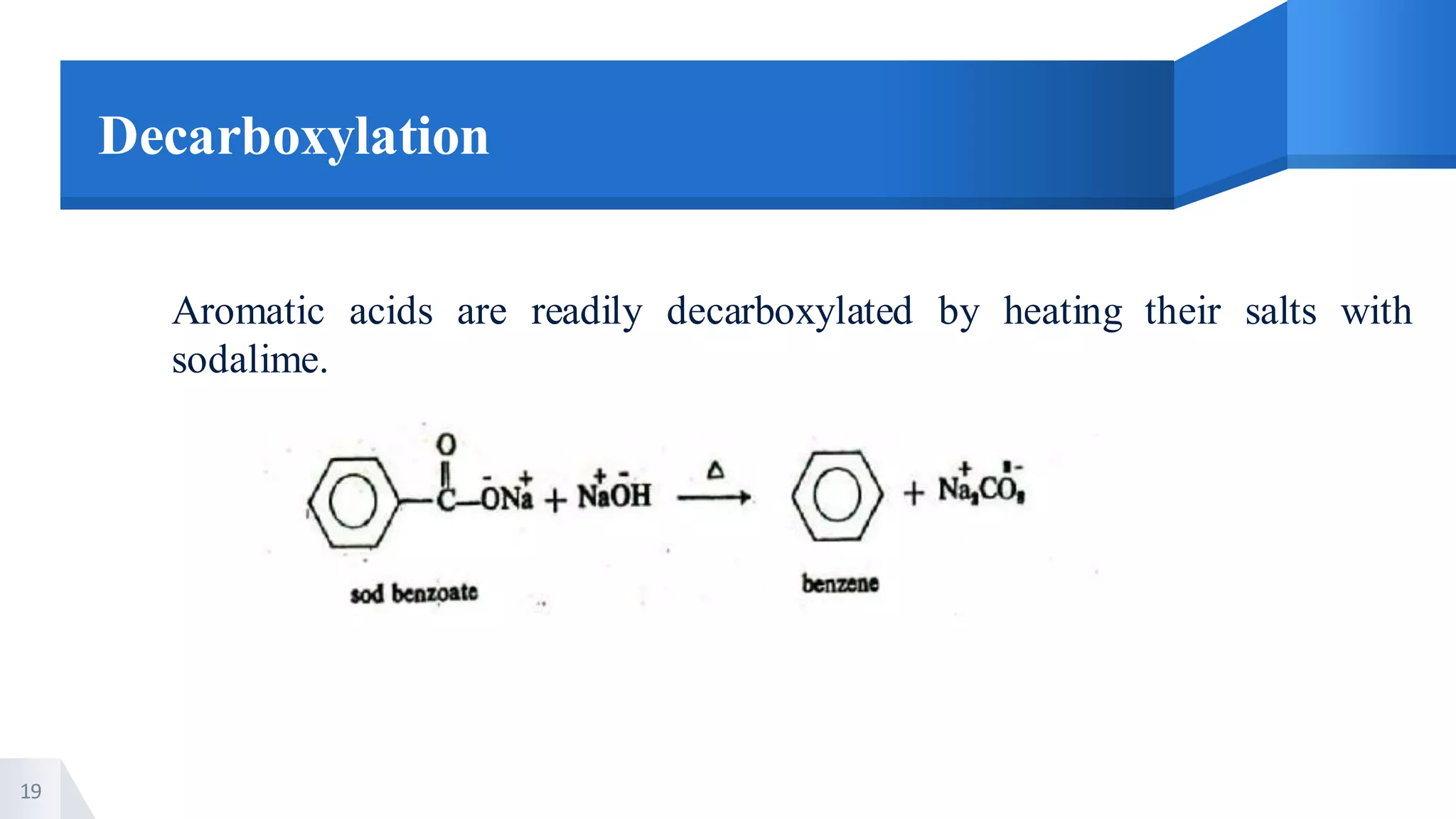
The document describes methods for preparing aromatic carboxylic acids and reactions of aromatic carboxylic acids. Six methods are provided for preparing aromatic carboxylic acids: 1) oxidation of primary alcohols and aldehydes, 2) hydrolysis of nitriles, 3) carbonation of grignard reagents, 4) oxidation of carbon side chains, 5) hydrolysis of trichloromethyl groups on benzene nuclei, and 6) Friedel-Craft reactions. Reactions of aromatic carboxylic acids that are discussed include esterification, anhydride formation, acid halide formation, acid amide formation, decarboxylation, reduction, and electrophilic substitution in benzene rings