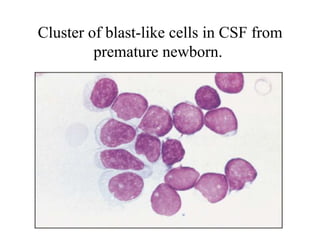
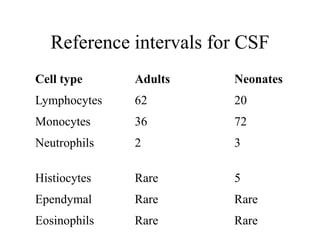
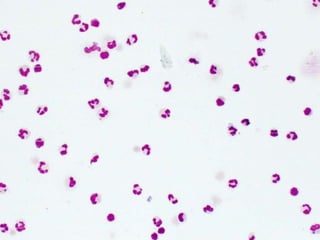
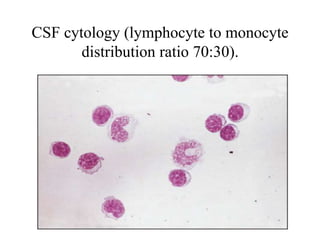
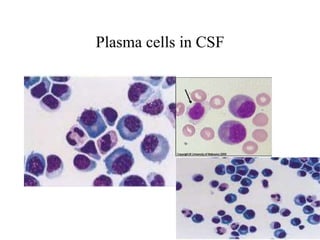
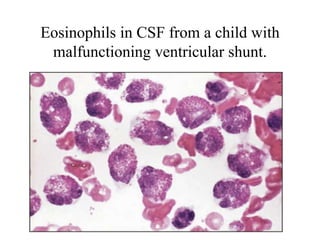
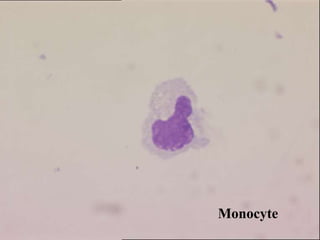
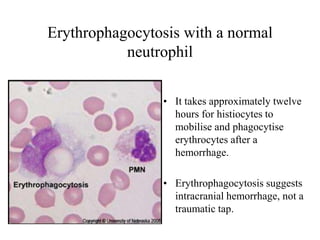
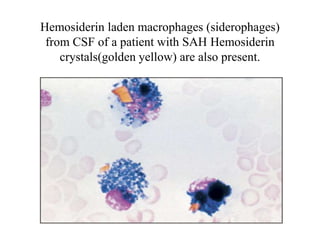
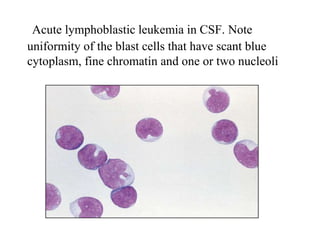
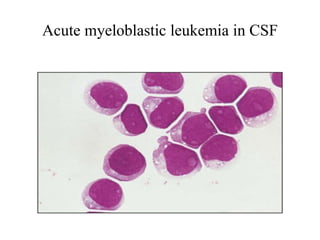
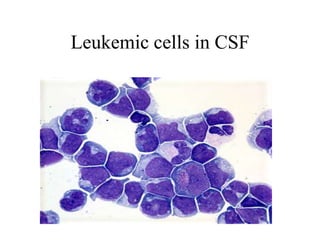
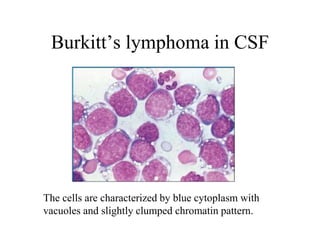
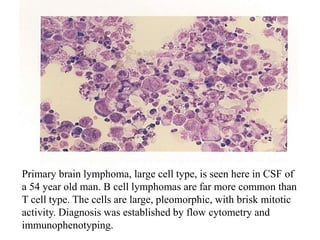
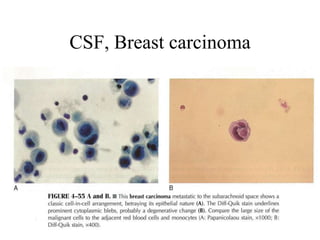
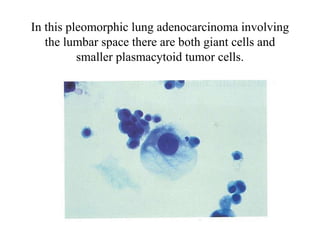
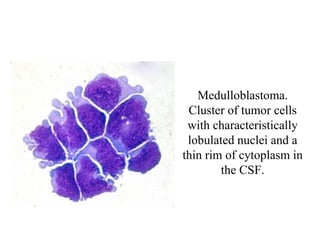
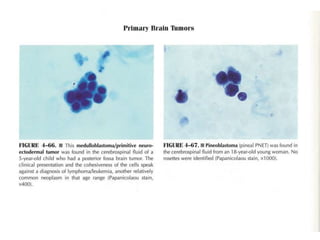
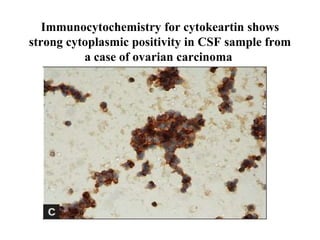
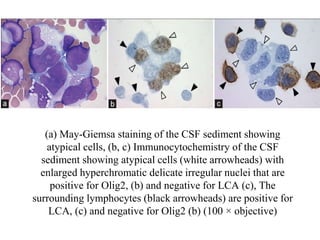
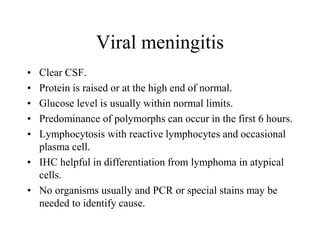
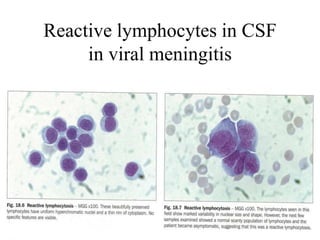
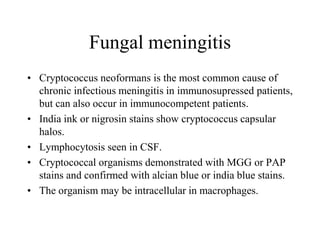
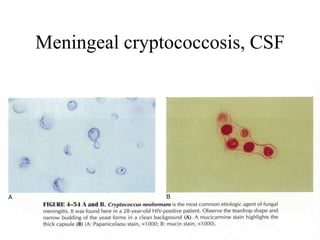
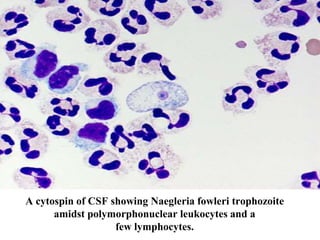
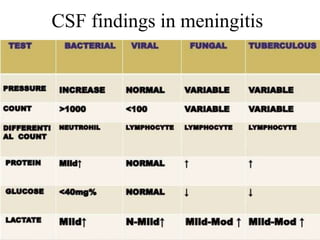
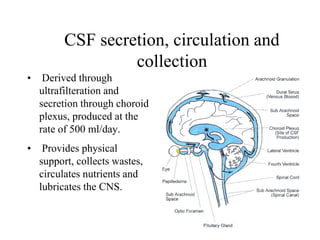
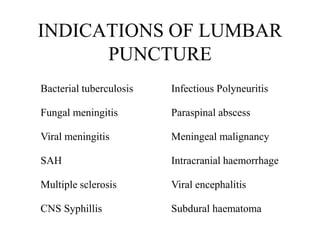
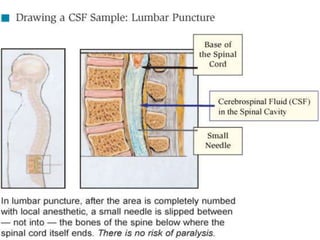
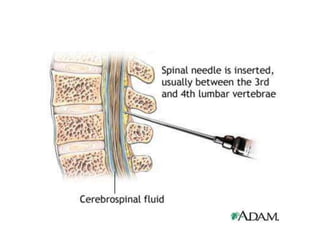
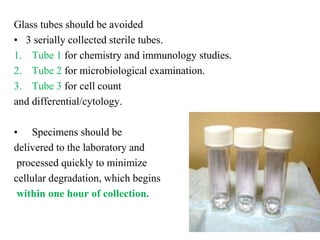
The document details a seminar on fluid cytology, specifically cerebrospinal fluid (CSF) and various bodily fluids. It covers methods of CSF collection, normal and abnormal CSF characteristics, laboratory tests, and differential diagnoses for various conditions, including infections and malignancies. Additionally, it discusses cytology findings and diagnostic markers relevant to identifying infections and tumors in the CSF.






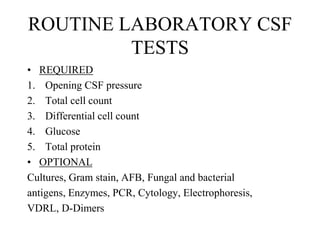
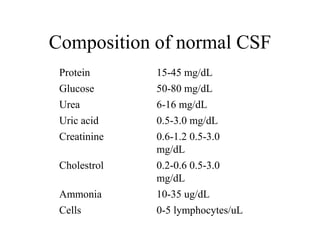
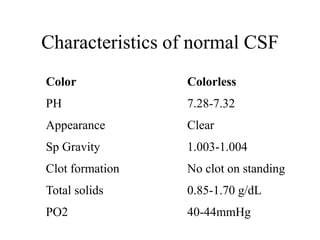












![Corrected WBC count:
WBC corr = WBC obs – WBC added
Where:
WBC added = WBCBLD x RBCCSF / RBCBLD
In the presence of normal peripheral blood RBC count, these
corrections amount to 1 WBC for every 700 RBC’c
Corrected protein:
TP added = [TP serum x (1 – HCT)] x RBCCSF/ RBCBLD
In the presence of normal serum protein, these corrections
amount to 8mg/dL protein for every 10,000 RBC’s/uL.](https://image.slidesharecdn.com/csfseminar-150127111214-conversion-gate01/85/Fluid-cytology-in-CSF-30-320.jpg)