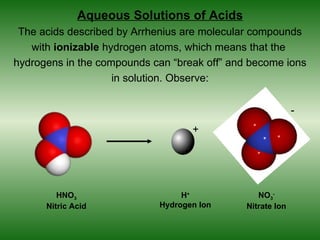
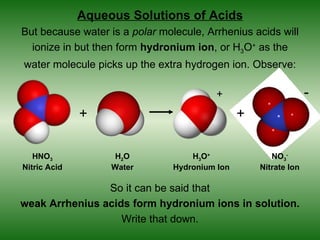
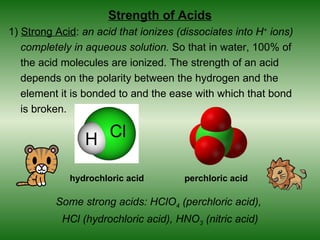
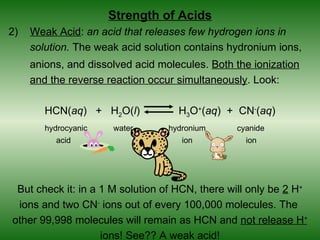
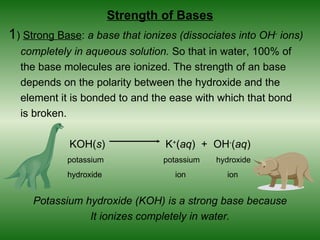
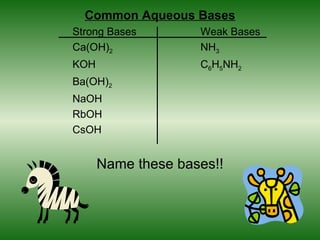
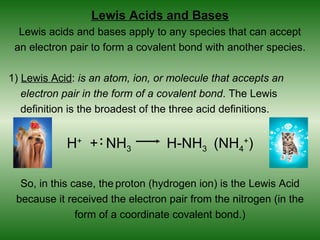
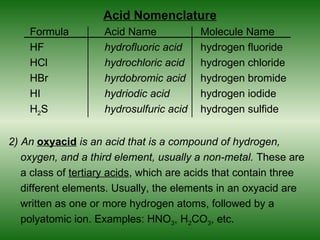
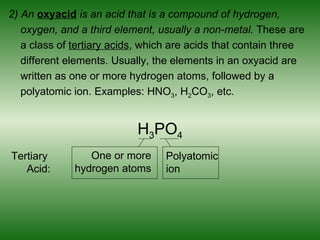
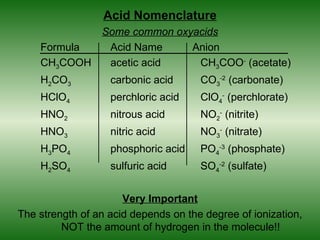
This document discusses acids and bases. It defines acids as compounds that increase the concentration of hydrogen ions (H+) in aqueous solution, and bases as compounds that increase the concentration of hydroxide ions (OH-). Strong acids and bases completely ionize in water, while weak acids and bases only partially ionize. Different acid theories are also introduced, including Arrhenius, Brønsted-Lowry, and Lewis definitions of acids and bases. Common strong and weak acids and bases are listed.



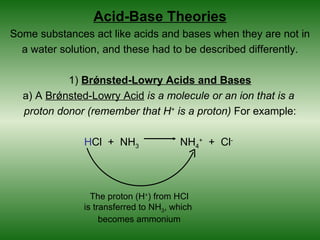
![Arrhenius Acids and Bases
An Arrhenius acid is a chemical compound that increases the
concentration of hydrogen ions, H+, in aqueous solution. In
other words, an acid will ionize in solution, producing H+ ions.
HCl (hydrochloric acid)
[H+] = hydrogen ion
concentration
H+ H+
free hydrogen Cl- [H+] = strong acid
ions Cl-
Cl- + H+ H+
H solution
Cl- H+ Cl-
H + [H+] = weak acid
- H
+
H+ Cl solution](https://image.slidesharecdn.com/chapter14-120513162906-phpapp01/85/Chapter-14-10-320.jpg)
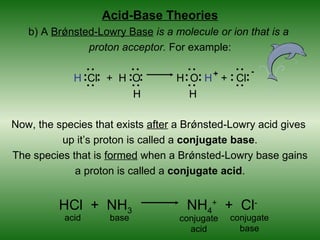
![Arrhenius Acids and Bases
An Arrhenius base is a chemical compound that increases the
concentration of hydroxide ions,OH-, in aqueous solution. In
other words, a base will ionize in solution, producing OH- ions.
NaOH (sodium hydroxide)
[OH-] = hydroxide ion
concentration
OH- OH +
-
free hydroxide [OH-] = strong base
Na+ Na
ions Na+ - OH- OH-
OH OH- Na+ solution
OH- Na
+
OH-
[OH-] = weak base
OH- Na+ solution](https://image.slidesharecdn.com/chapter14-120513162906-phpapp01/85/Chapter-14-11-320.jpg)