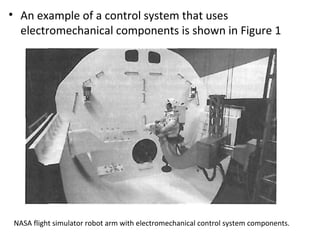
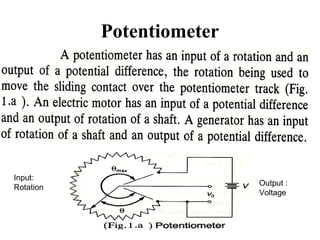
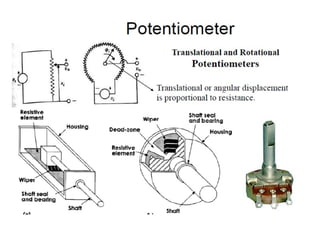
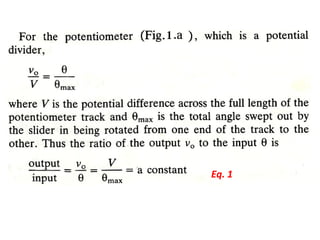
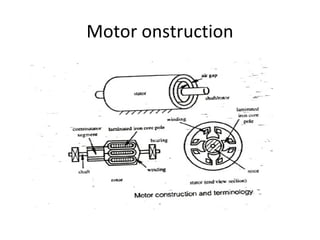
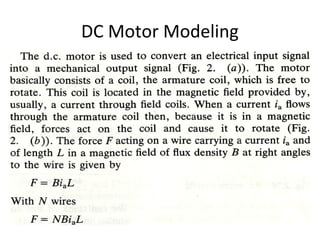
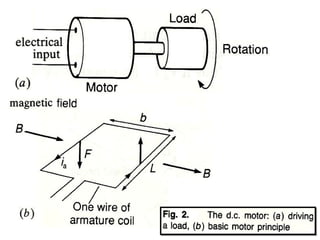
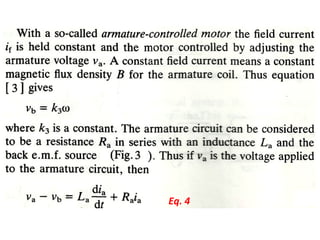
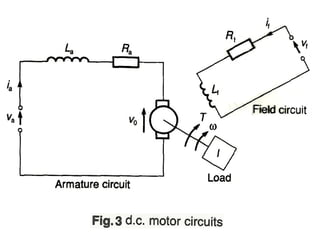
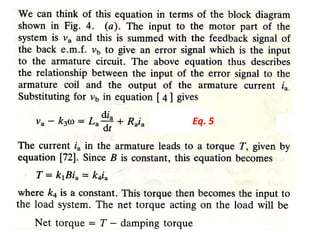
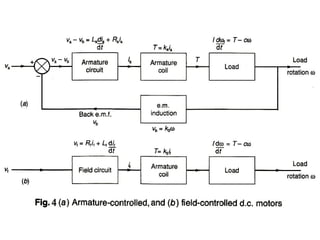
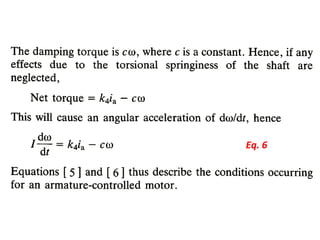
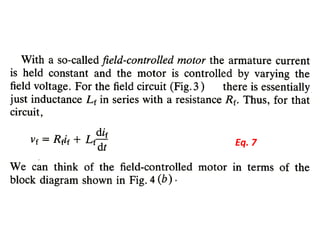
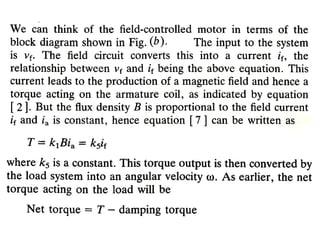
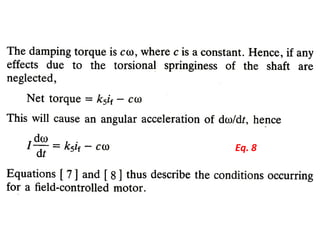
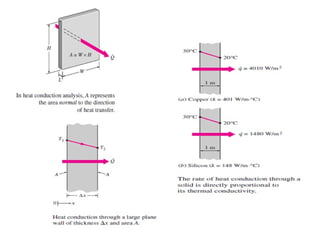
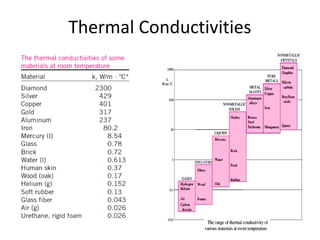
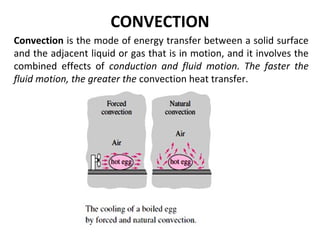
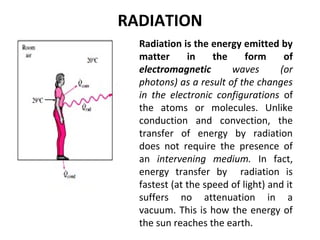
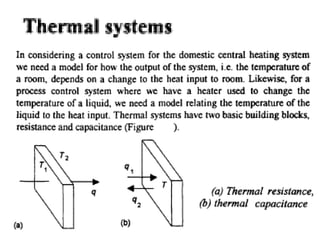
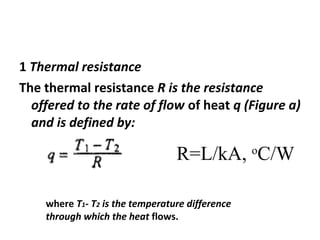
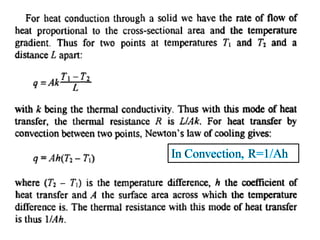
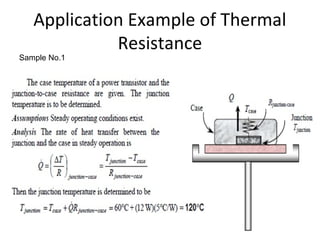
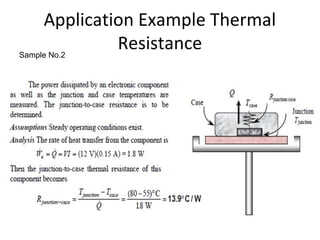
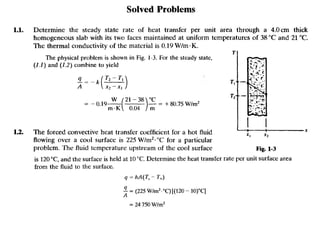
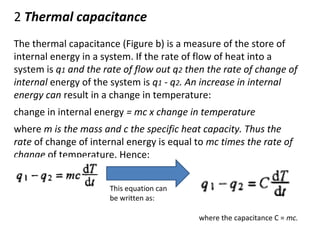
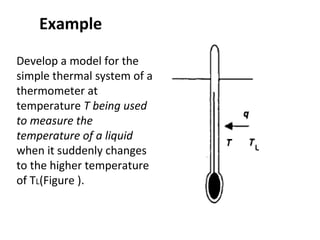
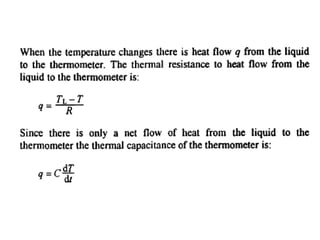
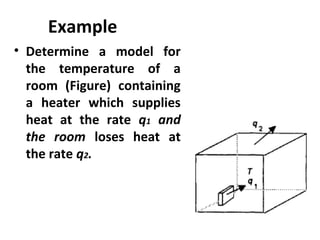
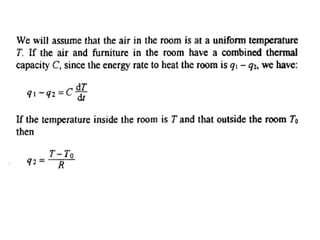
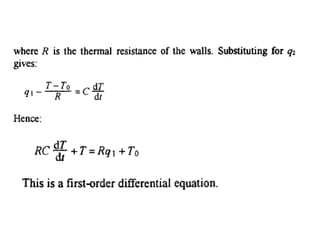
This document discusses electromechanical systems and their transfer functions. It provides examples of control systems using electromechanical components like a NASA robot arm. It also covers modeling of thermal systems, the three modes of heat transfer (conduction, convection and radiation), thermal resistance, thermal capacitance, and provides examples applying these concepts.